
Primary autoimmune inner ear disease in pregnancy
Introduction
Autoimmune inner ear disease (AIED) refers to a group of diseases in which hearing loss or loss of vestibular dysfunction results from an immune-mediated process. AIED can be broken down into primary and secondary types. Primary AIED includes pathology only involving the cochlea and vestibular organs, whereas secondary AIED arises in patients with multisystem autoimmune diseases that also involve the inner ear: such as Wegener granulomatosis, systemic lupus erythematosus, and Cogan’s disease.
McCabe first described AIED in 1979 (1). It was defined as bilateral sensorineural hearing loss (SNHL) that progressed over weeks to months and was responsive to immunosuppressive agents; he proposed an autoimmune mechanism. AIED is an important otological condition as it is one of the few causes of SNHL that is reversible.
While the literature contains a number of described cases of secondary AIED in pregnancy (2,3). This case represents the first case of primary AIED associated with pregnancy in the literature, where the patient had no prior autoimmune conditions and no systemic symptoms. It is of particular interest as it spans two pregnancies and provides multidisciplinary guidance for management and prognosis.
Case presentation
A 39-year-old woman presented with left SNHL at 22 weeks into her first pregnancy. The hearing loss had begun 5 weeks prior to presentation and had progressively worsened. She was otherwise fit and well. Her father has a history of rheumatoid arthritis and her brother has a history of ulcerative colitis.
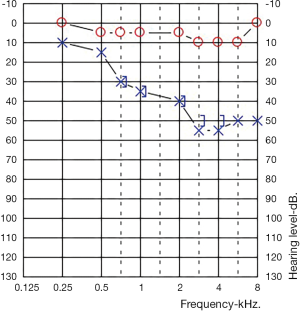
An audiogram demonstrated a left sided mild to moderate down sloping SNHL. Her right ear was within normal limits at all frequencies (Figure 1).
Under the guidance of rheumatology and her obstetrician, she was started on high dose prednisolone at 1 mg/kg (50 mg). An autoimmune blood screen was performed which demonstrated a strongly positive homogenous antinuclear antibody (ANA) titre of >1:2,560. She had never had a previous ANA for comparison. All other bloods tests were negative including anti-double stranded DNA antibodies, extractable nuclear antigen screen, rheumatoid factor, ANCA, lupus anticoagulant, anti-cardiolipin, HIV serology, syphilis serology, CMV serology and tuberculous screen. Magnetic resonance imaging of her brain and skull base was normal. Her Rheumatologist opted not to perform anti-heat shock protein testing given the potential for false-positive results.
After 2 weeks of high dose prednisolone, she was weaned over a month to 25 mg. The plan was to continue weaning by 5 mg per week to get her to the lowest dose of prednisolone with control of her symptoms. At 20 mg her hearing loss worsening and she was returned to 50 mg before starting the weaning regime again. From 31 weeks pregnant her hearing stabilised with 10 mg of prednisolone until her baby was safely delivered at 36 weeks.
Post-partum she was weaned off steroids over a 2-month period without any further decline in her hearing, however with the same mild-moderate hearing loss that she had at first presentation. Her serum ANA titre remained unchanged at 2 months post-partum.
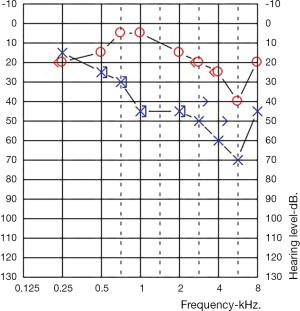
One-year post-partum she fell pregnant with her second pregnancy. At 24 weeks pregnant her right ear, contralateral to her previous SNHL, developed a mild down slowing SNH (Figure 2). This ear been normal throughout the prior pregnancy. The same screen bloods were performed with unchanged results (an ANA titre of >1:2,560, with all other tests normal). Her left ear was stable, unchanged from the end of her first pregnancy.
Once again, she was treated with 1 mg/kg prednisolone which was slowly weaned. This again required multiple dose adjusted as her hearing fluctuated, ultimately stabilising to a dose of 10 mg prior to delivery. She was again able to be wean at two months post-partum with stable hearing, but her audiometric thresholds did not improve.
Overall steroids were able to stabilize the hearing loss in both ears during both pregnancies, but did not reverse the hearing loss from presentation. During both pregnancies she had episodes where her hearing worsened during the weaning period, these deteriorations were improved by temporarily increasing the steroid dose.
Discussion
AIED is a rare disorder in both its primary and secondary forms. It has been estimated to account for less than 1% of hearing impairment or dizziness (4). The clinical hallmark of AIED is bilateral SNHL that progresses over weeks to months (1). Patients may also come to medical attention with unilateral hearing loss, and their course may fluctuate (4).
Pregnancy represents a time when women may become triggered to have autoimmune conditions. The literature describes a number of cases of secondary AIED in pregnancy, where a patient already has known autoimmune disease and goes on to develops SNHL in pregnancy (2,3). However, this is the first described case of patient with classic features of autoimmune ear disease without an associated systemic illness.
Pregnancy itself has not been shown to be associated with an increase in ANA, nor has it been associated with abnormalities of pregnancy (5). While the above patient’s positive ANA titer increased her rheumatologist’s suspicion that this was autoimmune in nature, it is the clinical course of this condition that supported the diagnosis of AIED.
Corticosteroids remain the standard of care for patients with primary AIED, and a prospective randomized clinical trial validated their use as empiric treatment (6). Initial therapy for adults consists of a therapeutic trial of prednisone 1 mg/kg/day, typically for 4 weeks. This must be started as soon as possible to minimise the chance of irreversible damage occurring. Therapeutic response may occur at any time during this period. Patients who do not respond are tapered rapidly over a week to 10 days. Patients who do respond are tapered slowly over 4 weeks to a maintenance dose of 10 to 20 mg/day. Steroid treatment may have to be continued for extended periods of time, and repeated cycles might be necessary for relapses (7). In the above patient steroids did not result in improvements of hearing from her initial presentation thresholds, but were successful able to treat subsequent audiometrically documented deterioration.
One of the key concerns with treating pregnant woman with high dose corticosteroids is that the safety of corticosteroid exposure in the first trimester of pregnancy is yet to be conclusively determined. The decision to put the above patient on long term steroids was made by her multidisciplinary team and after careful review of the literature.
Under normal conditions, metabolism of prednisolone by the human placenta likely reduces fetal exposure (8). Even though early reports have suggested an association between corticosteroid use and an increased risk of cleft lip and palate (9), more recent data have shown no increased risk of orofacial clefts or preterm delivery (10-12).
A prospective controlled cohort study of 311 pregnancies exposed to corticosteroids during at least the first trimester of pregnancy failed to identify an increased risk of birth defects, but did identify a 2-fold increase in preterm birth and reduction in birthweight (13).
Prolonged use of corticosteroids during later pregnancy has been associated with maternal adverse effects including hypertension, glucose intolerance, opportunistic infections, osteoporosis and ocular side effects (14). While ultimately systemic corticosteroid use is considered safe in most guidelines for the second and third trimester, the use of the lowest effective dose and monitoring of both maternal and foetal wellbeing by a multidisciplinary team is recommended.
An alternative to systemic steroids that was discussed with this patient was the use of intratympanic steroids. There are few clinical studies of intratympanic steroids for the treatment of AIED and there are no studies that look at the safety of intratympanic steroids in pregnancy. In a study involving 46 patients with various types of inner ear disease treated with intratympanic steroids, five were considered to have AIED (15). Four of the five patients with AIED treated with intratympanic steroids showed improvement in their hearing. Another study involving 37 patients with new-onset SNHL, included one patient with AIED whose fluctuating hearing loss was successfully treated with intratympanic steroids (9). While none of these cases specifically looked at the use of intratympanic steroids in pregnancy, these cases suggest that serial dosing of intratympanic steroids may represent an alternative for pregnant woman unable or unwilling to use systemic treatment.
A long-term follow-up of patients receiving steroid treatment for AIED showed an average of 9 years of treatment for those who were eventually able to be weaned (7). Ultimately, in the above patient, steroids were able to be successfully weaned within 2 months post-partum for both pregnancies without further decline in hearing.
Conclusions
This unique case is best understood as primary AIED associated with pregnancy. Ultimately oral steroids stabilized the hearing in both her ears during both pregnancies but did not improve her hearing from audiometry at presentation. Of note, systemic steroids were able to be completely weaned post-partum more rapidly than in typical cases of AIED.
Acknowledgments
Funding: S O’Leary is supported by a Practitioner Fellowship from the National Health and Medical Research Council (Australia).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.09.06). CI serves as an unpaid editorial board member of Australian Journal of Otolaryngology. SOL serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol 1979;88:585-9. [Crossref] [PubMed]
- Deane KD, Tyler KN, Johnson DW, et al. Susac syndrome and pregnancy: disease management. J Clin Rheumatol 2011;17:83-8. [Crossref] [PubMed]
- Kuczkowski J, Kozłowski J, Narozny W. Acute autoimmune sensorineural hearing loss in pregnant women with Leśniowski-Crohn disease. Otolaryngol Pol 2006;60:583-5. [PubMed]
- Bovo R, Aimoni C, Martini A. Immune-mediated inner ear disease. Acta Otolaryngol 2006;126:1012-21. [Crossref] [PubMed]
- Kiuttu J, Hartikainen AL, Mäkitalo R, et al. The outcome of pregnancy in antinuclear antibody-positive women. Gynecol Obstet Invest 1994;37:160-3. [Crossref] [PubMed]
- Harris JP, Weisman MH, Derebery JM, et al. Treatment of corticosteroid-responsive autoimmune inner ear disease with methotrexate: a randomized controlled trial. JAMA 2003;290:1875-83. [Crossref] [PubMed]
- Kanzaki J, Kanzaki S, Ogawa K. Long-term prognosis of steroid-dependent sensorineural hearing loss. Audiol Neurootol 2009;14:26-34. [Crossref] [PubMed]
- Addison RS, Maguire DJ, Mortimer RH, et al. Pathway and kinetics of prednisolone metabolism in the human placenta. J Steroid Biochem Mol Biol 1993;44:315-20. [Crossref] [PubMed]
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology 2000;62:385-92. [Crossref] [PubMed]
- Bay Bjørn AM, Ehrenstein V, Hundborg HH, et al. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am J Ther 2014;21:73-80. [Crossref] [PubMed]
- Czeizel AE, Rockenbauer M. Population-based case-control study of teratogenic potential of corticosteroids. Teratology 1997;56:335-40. [Crossref] [PubMed]
- Hviid A, Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ 2011;183:796-804. [Crossref] [PubMed]
- Gur C, Diav-Citrin O, Shechtman S, et al. Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reprod Toxicol 2004;18:93-101. [Crossref] [PubMed]
- Cassina M, Fabris L, Okolicsanyi L, et al. Therapy of inflammatory bowel diseases in pregnancy and lactation. Expert Opin Drug Saf 2009;8:695-707. [Crossref] [PubMed]
- Silverstein H, Choo D, Rosenberg SI, et al. Intratympanic steroid treatment of inner ear disease and tinnitus (preliminary report). Ear Nose Throat J 1996;75:468-71, 474, 476 passim. [PubMed]
Cite this article as: Hill FCE, Iseli C, O’Leary S. Primary autoimmune inner ear disease in pregnancy. Aust J Otolaryngol 2018;1:23.


