A state-wide survey of disinfection techniques for nasendoscopies in Queensland ENT out-patient departments
Introduction
Flexible nasendoscopy is an important part of the diagnostic process in Otorhinolaryngology. It provides immediate, direct and accurate visualisation of the upper aerodigestive tract and is a vital tool in various ear, nose and throat (ENT) emergencies and routine clinical practice. Flexible nasendoscopies come in close contact with mucous membranes of the upper aerodigestive tract. Therefore, appropriate and effective disinfection is vital to prevent iatrogenic infection and cross contamination.
Flexible nasendoscopies are considered semi-critical instruments and high level disinfection (HLD) has been recommended to prevent nosocomial infections. The lack of effective disinfection and sterilisation can lead to nosocomial outbreaks which have been reported with the use of gastrointestinal endoscopes and bronchoscopes (1). HLD is tuberculocidal, virucidal, fungicidal, bactericidal and sporicidal in some but not all bacterial endospores which are not covered (2). Various types of HLD are available including 2% glutaraldehyde, 7.5% hydrogen peroxide, chlorine dioxide and 0.2% peracetic acid. Disinfection techniques include immersion or wipes with liquid HLD, automated endoscope reprocessors (AEMs) and disposable endosheaths.
Specialities such as gastroenterology have established clear guidelines for cleaning and disinfecting fibre-optic endoscopes (3). To date, there is no published data or comprehensive guideline for disinfection of nasendoscopies in otorhinolaryngology in Australia. This study aims to demonstrate the current disinfection practice of nasendoscopies in Queensland.
Methods
A questionnaire was designed to establish the methods used for disinfection of flexible nasendoscopies within ENT departments in Queensland. All fourteen ENT departments within the state public health framework were invited to complete an online questionnaire hosted by an online survey program—Survey Monkey. Questions addressed the disinfection technique used, location of cleaning, specific HDL or device used, time taken for disinfection and record of reprocessing. Additionally, information on the designated staff members trained in disinfection and techniques used in high risk patient groups (HIV, hepatitis B and C and pulmonary tuberculosis) were also collected. The results of each questionnaire were compiled by Survey Monkey. The results are presented as frequencies and percentages.
Results
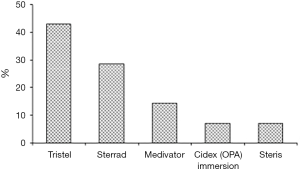
Fourteen questionnaires were satisfactorily completed. All departments reported to have internal guidelines on cleaning and storage practices for nasendoscopies. Manual disinfection technique was used in 7 hospitals (50 percent) and AEMs were used in 7 hospitals (50 percent). Manual disinfection with Tristel (chlorine dioxide based disinfectant wipes) was used in 6 hospitals (42.9 per cent), and immersion in Cidex OPA (0.55% ortho-phthalaldehyde) was used in one hospital (7.1 percent) (see Figure 1). None of the departments used disposable endosheaths. The same disinfection technique was used after hours and in high-risk patients (HIV, hepatitis B, hepatitis C, pulmonary tuberculosis) in all hospitals. Disinfection was performed at the ENT outpatient department in 9 hospitals (64.3 percent) and the 5 (35.7 percent) remaining hospitals processed their scopes in the Central Sterile Services Department (CSSD) (see Figure 2). All staff members who performed disinfection in the outpatient department were trained in the respective technique used. A record of nasendoscopy maintenance and processing were kept in all fourteen hospitals. A record of patient name and patient identification number, date of procedure and serial number of both the nasendoscopy and the disinfectant were kept by all departments.
Discussion
Flexible nasendoscopy has superseded traditional indirect laryngoscopy as it allows rapid and clear visualisation of the upper aerodigestive tract. Its use has become ubiquitous in current ENT practice and is widely used in the out-patient department, wards and the emergency setting. Owing to their routine use and close contact with mucous membranes, disinfection of flexible nasendoscopies is of paramount importance as failure to employ appropriate methods could lead to nosocomial outbreaks.
Flexible nasendoscopies are classified as semi-critical instruments and require HLD to prevent nosocomial infections. Disinfection techniques commonly used for nasendoscopies are manual immersion or wipes with liquid HLD, AERs and disposable sheaths. This study demonstrates there is considerable variation in technique, with the AER and manual disinfection being equally common methods used in public hospitals and health services across Queensland. The similar lack of uniformity has also been demonstrated abroad. A national survey performed in the UK reported chemical immersion as the most common technique, followed by alcohol wipe and disposable plastic sheath (4). Similarly, a survey in South Africa revealed that the majority of otolaryngologists used manual disinfection techniques with immersion or wipes with HLDs (5).
HLD of flexible nasendoscopies can be achieved through various methods. To date, the single most efficacious technique has yet to be established as each method has its own advantages and disadvantages. The current ‘gold standard’ for decontamination of nasendoscopy is considered to be the AER, which is an automated washer within a centralised decontamination unit (6). The main advantage of AERs is that they allow standardised decontamination compared to manual disinfection, which is often inconsistent and liable to human error (7,8). This technique also allows regular testing and revalidation of the decontamination process (9). However, mechanical cleaning, leak testing and use of enzymatic detergent are still recommended before the use of an AER (10,11). Other disadvantages of AERs are the high installation and maintenance costs (9,12). Despite these costs, a study comparing the cost effectiveness between AERs and chlorine dioxide wipes, showed that AERs were more cost effective in the long term (12).
Chlorine dioxide based wipes are another emerging method of disinfection. Our study showed chlorine dioxide based wipes were the second most popular disinfection technique used after AERs in ENT departments across Queensland. Chlorine dioxide has been proven to be effective against bacteria, fungi and Mycobacterium terrae (12). It is also active against hepatitis C virus and HIV after 30 seconds of contact time (12). An in vitro study comparing the efficacy of chlorine dioxide wipes with automated washers showed a significantly lower Staphylococcus epidermidis growth in the chlorine dioxide group (12). Another recent study by Hitchcock et al. showed that the microbiological efficacy of Tristel Trio Wipes (chlorine dioxide based) were comparative to Perasafe (0.2% peracetic acid) and Cidex OPA (0.55% ortho-phthalaldehyde) (13). Additionally, the turnaround time and costs were significantly reduced with Tristel Trio Wipes compared to PeraSafe and Cidex OPA (13). Apart from microbial efficacy, chlorine dioxide based wipes do not incur any installation costs and disinfection can be performed immediately after use. However, studies have shown manual decontamination is liable to human error and highly inconsistent in terms of technique and duration (4,8,14).
Recently, disposable endosheaths have become another option for reprocessing nasendoscopies. These are clear material single use devices designed to fit tightly over the tip of the flexible endoscope to provide a mechanical barrier against contamination. These sheaths offer the advantage of increasing the patient throughput by reducing the endoscope downtime associated with HLD. However, there have been concerns regarding the false level of assurance, protection and patient safety, as sheaths may tear or be breached during use. Currently, there are two publications that validate the reliability of the sheath with nasendoscopies. Alvarado et al. showed that polyurethane sheath combined with enzymatic detergent cleaning and disinfection with 70% ethanol provided a reliable decontamination (15). Another study by Elackattu et al. reported similar contamination rates between endosheaths alone and immersion with high-level disinfectant, the average time spent using sheath was 89 seconds compared to 14 minutes with the immersion method (16). Given the rapid reprocessing time, this technique could be useful particularly in busy departments with high turnover of nasendoscopies.
Our study demonstrated that hospital staffs were trained in the respective disinfection technique used at each facility. High standards of training are imperative to maximise nasendoscopy reprocessing efficiency and to ensure long-term endoscope viability. Regardless of the technique used, staff members should be trained in additional practices such as immediate manual cleaning, leak testing and cleaning with an enzymatic agent. Leak testing is critical as submersion of a non-intact endoscope into a liquid disinfectant could lead to damage of the instrument. Cleaning with an enzymatic agent should also be performed before any accumulated secretions dry.
To date, there is insufficient evidence to demonstrate the superiority in efficacy of any one technique over the others. Cost, convenience and reprocessing time are the main factors that differ between the various methods. Hence, the type of technique used should be tailored to the number of scopes, patient throughput, availability of staff and funding available to each department. There is no consensus regarding the immersion time, efficacy of chemical agents, training of staff members and protocols for high-risk patients in otorhinolaryngology. In contrast, specialities such as gastroenterology have well designed guidelines for endoscope disinfection. Similar protocols should thus be established within otorhinolaryngology that outlines reprocessing standards required for all types of disinfection methods.
Conclusions
This study demonstrates that the reprocessing of nasendoscopies across public ENT departments in Queensland varies considerably. The publication of national or state based guidelines would enable standardisation of reprocessing services and improve safety for both patients and staff members within otorhinolaryngology.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.10.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med 1993;118:117-28. [Crossref] [PubMed]
- Kanagalingam J, Zainal A, Georgalas C, et al. The disinfection of flexible fibre-optic nasendoscopes out-of-hours: confidential telephone survey of ENT units in England. J Laryngol Otol 2002;116:817-22. [Crossref] [PubMed]
- DiMarino AJ, Bond WW. Flexible gastrointestinal endoscopic reprocessing. Gastrointest Endosc 1996;43:522-4. [Crossref] [PubMed]
- Banfield GK, Hinton A. A national survey of disinfection techniques for flexible nasendoscopes in UK ENT out-patient departments. J Laryngol Otol 2000;114:202-4. [Crossref] [PubMed]
- Lubbe DE, Fagan JJ. South African survey on disinfection techniques for the flexible nasopharyngoscope. The Journal of Laryngology & Otology 2003;117:811-4. [Crossref] [PubMed]
- Swift A. editor. Guidance on the decontamination and sterilization of rigid and flexible endoscopes. ENT UK trading as British Academic Conference in Otolaryngology (BACO) and British Association of Otorhinolaryngology, Head & Neck Surgery (BAO-HNS, 2010), 2010.
- Kaczmarek RG, Moore RM, Mccrohan J, et al. Multi-state investigation of the actual disinfection/sterilization of endoscopes in health care facilities. Am J Med 1992;92:257-61. [Crossref] [PubMed]
- Muscarella LF. Inconsistencies in endoscope-reprocessing and infection-control guidelines: the importance of endoscope drying. Am J Gastroenterol 2006;101:2147. [Crossref] [PubMed]
- Collins WO. A review of reprocessing techniques of flexible nasopharyngoscopes. Otolaryngol Head Neck Surg 2009;141:307-10. [Crossref] [PubMed]
- Alvarado CJ, Reichelderfer M. APIC guideline for infection prevention and control in flexible endoscopy. Am J Infect Control 2000;28:138-55. [Crossref] [PubMed]
- Thompson G, Buchanan Y, Alfa M. Guidelines for infection prevention and control in endoscopy. Manitoba Advisory Committee on Infectious Diseases [published 2000]. Available online: http://cpsm.mb.ca/cjj39alckF30a/wp-content/uploads/NHMSF%20Appendix%20F%20Endoscopy%20Guidelines.pdf
- Phua CQ, Mahalingappa Y, Karagama Y. Sequential cohort study comparing chlorine dioxide wipes with automated washing for decontamination of flexible nasendoscopes. J Laryngol Otol 2012;126:809-14. [Crossref] [PubMed]
- Hitchcock B, Moynan S, Frampton C, et al. A randomised, single-blind comparison of high-level disinfectants for flexible nasendoscopes. J Laryngol Otol 2016;130:983-9. [Crossref] [PubMed]
- Muscarella LF. Prevention of disease transmission during flexible laryngoscopy. Am J Infect Control 2007;35:536-44. [Crossref] [PubMed]
- Alvarado CJ, Anderson AG, Maki DG. Microbiologic assessment of disposable sterile endoscopic sheaths to replace high-level disinfection in reprocessing: a prospective clinical trial with nasopharygoscopes. Am J Infect Control 2009;37:408-13. [Crossref] [PubMed]
- Elackattu A, Zoccoli M, Spiegel JH, et al. A comparison of two methods for preventing cross-contamination when using flexible fiberoptic endoscopes in an otolaryngology clinic: Disposable sterile sheaths versus immersion in germicidal liquid. Laryngoscope 2010;120:2410-6. [Crossref] [PubMed]
Cite this article as: Chandran D, Lomas J, Anderson J, Green M, McKenzie JL, Grigg R. A state-wide survey of disinfection techniques for nasendoscopies in Queensland ENT out-patient departments. Aust J Otolaryngol 2018;1:28.