
A rare case of toxigenic diphtheria tonsillitis resistant to penicillin causing sepsis and death
Introduction
Diphtheria is a rare, contagious and potentially life threatening acute bacterial infection caused by toxigenic and non-toxigenic strains of Corynebacterium diphtheriae (1). Previously a source of significant morbidity and mortality in the Australian population, the implementation of public health initiatives including greater community awareness and most notably, active immunization programs lead to a marked decline in incidence, and near complete eradication by the 1960s (2). A minority of cases continue to be reported on yearly basis, restricted to unimmunized individuals travelling to, or having contact with travelers from countries where diphtheria remains endemic (3), and in subpopulations in the Northern Territory (particularly indigenous Australians), where non-toxigenic strains remain endemic (4). Additionally, there has been an emerging trend of adult infections postulated to be the consequence of childhood immunization reducing the clinical incidence of, and circulation of the toxigenic diphtheria strain, preventing the opportunity to acquire natural immunity or boost vaccine induced immunity (5). On account of the aforementioned public health initiatives and wide access to penicillin-based therapy, diphtheria-related mortality in Australia is exceedingly rare with the last reported occurrence in 1992 (5). Herein, we describe the first subsequent death from toxigenic Corynebacterium diphtheriae and first reported case of penicillin-resistance.
Case presentation
A previously healthy, unimmunized, non-indigenous 22-year-old female presented to the Emergency Department of Princess Alexandra Hospital (Brisbane, Australia) with a 6-day history of progressive odynophagia, dysphagia and reduced oral intake despite analgesia and antibiotics (penicillin). She had been reviewed twice previously throughout the course of her illness and had received intravenous benzylpenicillin and dexamethasone for acute tonsillitis (in addition to her outpatient oral therapy). Serological testing for Epstein-Barr virus was negative. On examination, apart from a mild tachycardia (108 beats per minute), her observations were within normal parameters. She was dysphonic, but conversing in full sentences, not stridulous and swallowing her saliva. Oral examination revealed grade 2+ erythematous tonsils with a pseudomembranous coating. Flexible nasendoscopy demonstrated extension of the pseudomembrane to the oro- and hypopharynx, supraglottis and vocal cords bilaterally. She was admitted for intravenous antibiotic (benzylpenicillin and metronidazole) and dexamethasone therapy.
The following day, the patient deteriorated with increased work of breathing, stridor and worsening hypoxia (SaO2 88% on 10 L of oxygen) necessitating emergency intubation and transfer to the intensive care unit. Intravenous therapy was continued as previous. On the third day of admission, her ventilator requirements had increased and bilateral lower lobe pulmonary consolidation was noted. Urgent bronchoscopy revealed a pseudomembranous coating of the lower respiratory tract extending into subsegmental bronchi (Figure 1). Tissue biopsies taken from the pseudomembranous oropharynx on admission cultured positive for Corynebacterium diphtheriae, however sensitivities were pending (Figure 2). Following test dosing, diphtheria antitoxin was administered. Her condition continued to worsen on the fourth day with problematic oxygenation secondary to pseudomembrane sloughing, endotracheal tube and bronchial plugging and worsening sepsis manifesting as acute renal and hepatic failure and increasing inotropic requirements. Ticarcillin was commenced empirically following consultation with infectious diseases. On days 5 and 6, end organ failure worsened and she developed toxin-induced cardiomyopathy and nephropathy. At this time, it was discovered that this strain of C. diphtheriae was resistant to penicillin. Meropenem and lincomycin were commenced, however despite maximal efforts, her condition continued to deteriorate, and she passed away shortly thereafter of acute cardiac failure secondary to complete heart block and refractory ventricular arrhythmias. Autopsy confirmed significant myocardial dystrophic, necrotic and sclerotic alterations. Additional relevant autopsy findings included disseminated mucosal ulceration of the pharynx, larynx, trachea and main bronchi with pseudomembranous coating. Mucosal erythema and satellite coating of smaller bronchi was also noted in association with pulmonary haemorrhage and bronchial plugging with fibrinopurulent exudate.
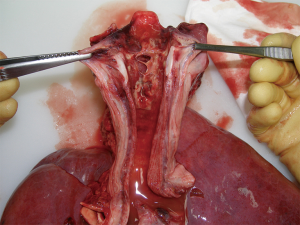
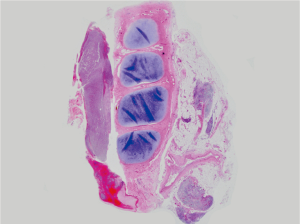
Discussion
Corynebacterium diphtheriae is gram positive, non-motile, unencapsulated bacillus transmitted via direct contact or respiratory droplets (1,6). Toxin production is dependent on lysogenic integration of one of a family of corynebacteriophages carrying the structural gene for diphtheria toxin (7). Lysogenic conversion from non-toxigenic to toxigenic strain can occur in situ or in vitro (7). Toxigenic strains are responsible for most pharyngeal, respiratory, cardiac, neurological and systemic sequelae, whilst non-toxigenic strains manifest predominantly as cutaneous infections (1,6). However, a recent increase in invasive non-toxigenic infections suggests that C. diphtheriae may possess additional virulence factors other than toxin-linked molecular mechanisms (8).
Early clinical recognition is paramount to successful management. As highlighted in this case, pharyngeal diphtheria presents with findings of pharyngitis, fever, cervical lymphadenitis (bull-neck diphtheria) and airway obstruction secondary to gray-green pseudomembrane formation (fibrin, bacteria and inflammatory cells) (7). Pseudomembrane coating may be difficult to distinguish from the more common exudative plaques associated with bacterial and viral tonsillitis (e.g., Epstein-Barr virus). Cutaneous diphtheritic lesions are usually covered by a gray-brown pseudomembrane (7). Disease progression signifies toxin penetrance into myocardium or peripheral neural tissue, at which stage the effects are rarely reversible (7).
Management of acute toxigenic pharyngeal diphtheria entails isolation and droplet precaution interactions, health department notification, diphtheria antitoxin administration, penicillin-based antimicrobial therapy and contact tracing. Prompt passive immunization with diphtheria antitoxin is most effective in reducing mortality which can reach 20–30% (7,9). Unfortunately, in our case, the disease had significantly progressed by the time of administration and toxin-mediated systemic effects could not be reversed or suppressed. Penicillin is the mainstay of therapeutic and prophylactic treatment. Resistance, as demonstrated in our case is rare and was unexpected, and this in combination with the patient’s lack of underlying immunity lead to her ultimately succumbing to the infection. Although penicillin-resistance has been reported in non-toxigenic strains of C. diphtheriae previously (10,11), to the best of our knowledge, this is the first reported case of penicillin-resistant toxigenic C. diphtheriae.
In recent times, objection to immunization and parental refusal to adhere to recommended health authority guidelines has come to the fore in the Australian media and public. Although recent analysis has only demonstrated a small increase in registered or unregistered vaccination objection (to 3.3%) (12), the issue has been of sufficient significance so as to prompt multiple federal and state government initiatives (13). However, these enterprises are restricted to the pediatric population and consequently, vast numbers of adults remain under-vaccinated (14). This case demonstrates the importance of childhood and regular adult booster immunization against diphtheria, which in our case, may have prevented the development of, or limited the extent of infection. Additionally, the importance of routine questioning of immunization status and consideration of diphtheria amongst the differential diagnoses is highlighted. However, a complete reliance on immunization history is thwart with danger as immune titers to Corynebacterium diphtheriae toxin may be suboptimal in previously immunized individuals (15). Furthermore, with the benefit of hindsight, additional interventions to be considered in such atypical and refractory cases of tonsillitis include obtainment of pharyngeal swabs earlier in the disease course (in the context of multiple emergency department representations and negative serology for Epstein-Barr virus), and empiric treatment with broader spectrum antimicrobial agents through consultation with infectious diseases.
Conclusions
Immunization is fundamental in preventing diphtheria-related morbidity and mortality. Clinicians must be wary of emerging penicillin-resistance and prompt antibiotic escalation considered if clinical deterioration occurs and specimen sensitivities are unavailable.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.10.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The patient died within 6 days of hospital admission, and the family/next of kin of the patient were no longer contactable. Institutional human research ethics committee approval was obtained for this study (HREC/11/QPAH/274).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee HJ, Choi JH. Tetanus-diphtheria-acellular pertussis vaccination for adults: an update. Clin Exp Vaccine Res 2017;6:22-30. [Crossref] [PubMed]
- Kilham H, Benn R. Diphtheria--the Australian perspective. Commun Dis Intell 1997;21:164-5. [PubMed]
- Abdul Rahim NR, Koehler AP, Shaw DD, et al. Toxigenic cutaneous diphtheria in a returned traveller. Commun Dis Intell Q Rep 2014;38:E298-300. [PubMed]
- Gordon CL, Fagan P, Hennessy J, et al. Characterization of Corynebacterium diphtheriae isolates from infected skin lesions in the Northern Territory of Australia. J Clin Microbiol 2011;49:3960-2. [Crossref] [PubMed]
- Gidding HF, Burgess MA, Gilbert GL. Diphtheria in Australia, recent trends and future prevention strategies. Commun Dis Intell 2000;24:165-7. [PubMed]
- Nandi R, De M, Browning S, et al. Diphtheria: the patch remains. J Laryngol Otol 2003;117:807-10. [Crossref] [PubMed]
- Murphy JR. The diphtheria toxin structural gene. Curr Top Microbiol Immunol 1985;118:235-51. [Crossref] [PubMed]
- Zasada AA, Baczewska-Rej M, Wardak S. An increase in non-toxigenic Corynebacterium diphtheriae infections in Poland--molecular epidemiology and antimicrobial susceptibility of strains isolated from past outbreaks and those currently circulating in Poland. Int J Infect Dis 2010;14:e907-12. [Crossref] [PubMed]
- Eskola J, Lumio J, Vuopio-Varkila J. Resurgent diphtheria--are we safe?. Br Med Bull 1998;54:635-45. [Crossref] [PubMed]
- FitzGerald RP, Rosser AJ, Perera DN. Non-toxigenic penicillin-resistant cutaneous C. diphtheriae infection: a case report and review of the literature. J Infect Public Health 2015;8:98-100. [Crossref] [PubMed]
- Fricchione MJ, Deyro HJ, Jensen CY, et al. Non-Toxigenic Penicillin and Cephalosporin-Resistant Corynebacterium diphtheriae Endocarditis in a Child: A Case Report and Review of the Literature. J Pediatric Infect Dis Soc 2014;3:251-4. [Crossref] [PubMed]
- Beard FH, Hull BP, Leask J, et al. Trends and patterns in vaccination objection, Australia, 2002-2013. Med J Aust 2016;204:275. [Crossref] [PubMed]
- Ward K, Quinn H, Bachelor M, et al. Adolescent school-based vaccination in Australia. Commun Dis Intell Q Rep 2013;37:E156-67. [PubMed]
- Menzies RI, Leask J, Royle J, et al. Vaccine myopia: adult vaccination also needs attention. Med J Aust 2017;206:238-9. [Crossref] [PubMed]
- Epp C, Aoki FY. Fatal diphtheria in an older woman. Can Med Assoc J 1985;132:663-4. [PubMed]
Cite this article as: Floros P, Gunaratne DA, Chang A, Coman WB. A rare case of toxigenic diphtheria tonsillitis resistant to penicillin causing sepsis and death. Aust J Otolaryngol 2018;1:29.


