
How I do it—endoscopic composite cartilage graft tympanoplasty
Introduction
The management of anterior, subtotal or total tympanic membrane perforations can be challenging through a transcanal approach, especially through a narrow canal or with a prominent anterior overhang (1). Endoscopic views can provide a wider and more detailed image of the entire annulus by placing the objective lens in the external ear canal. This improves the visualization and facilitates the access to the anterior annulus, which can be often limited.
This study discusses the method and short-term results of transcanal endoscopic composite cartilage tympanoplasty for anterior and total perforations by customizing the steps previously described in the EES technique (2,3). In addition, we aim to present how modifying some of the steps in graft harvesting and placement better capitalize on the endoscopic view for the securement of the graft.
Methods
A retrospective analysis of the charts of patients undergoing tympanoplasty under this technique from 2010–2017 was performed by three different otologists in private and public hospitals across Sydney, Australia. All the perforations were medium to large or subtotal in size. Patients of all ages were included. Presence of cholesteatoma was an exclusion criterion. Audiological results are based on a smaller subset, as some patients failed to provide these results. Results were analyzed using SPSS/IBM® statistics software, Armonk, NY, USA. Paired sample analysis utilized Student t test. Statistical significance was obtained through the v2 test, and when appropriate. Fisher exact test was applied. An alpha value was considered significant when P<0.05.
Surgical technique
This technique has been standardized among the surgeons involved in this paper, however slight differences exist on a case by case basis. This is a result of the principle on which this technique is based: to adapt to the patient’s intrinsic anatomy and size and location of the perforation as keys to a favorable outcome.
A meticulous ear canal debridement and dewaxing as well as careful trimming of the external auditory canal (EAC) hairs help maintain a clean endoscope lens and prevent implantation of epithelium into the middle ear cavity by decreasing the chance of pushing debris into the middle ear throughout the procedure (2-5).
Optimization of hemostasis
Adequate hemostasis impacts the quality of the image as well. Total intravenous anesthesia (TIVA), that maintains the patient hypotensive and bradycardic, a reverse Trendelenburg position, local infiltration of the ear canal and graft donor site with a mixture of epinephrine and local anesthetics as well as the placement of neurosurgical patties soaked in 1:1,000 epinephrine in the ear canal and against the tympanic membrane (TM) for 5–10 minutes are good strategies to minimize bleeding throughout the procedure. The local anesthetic mixtures vary between surgeons (1:100,000 to 1:70,000, typically). It is advisable to use the mixture that the surgeon feels comfortable with after considering the general health of the patient and their previous experience (4,5).
Preparation of the tympanic membrane
The perforation’s edge is freshened and a tympanomeatal flap (TMF) raised. The size of the TMF is customized by the extension of the perforation and surgeon’s preference in each particular case. Conditions of the ear canal skin, anatomical hurdles or a need to increment space for instrument manipulation may increase or decrease the size of the graft. Typically, a wider extension is usually required for an anterior perforation, which makes the approach and graft placement more comfortable. In general, an anterosuperior extension is favored, extending beyond the lateral process of the malleus handle (1). As the annulus is lifted off its bony groove, the chorda tympani with the posterior malleolar ligament crossing over it are identified. The flap is raised superficial to this ligament opening Prussak’s space.
Separation of the TM from the malleus handle and umbo is an essential aspect of the procedure. The periosteum over its posterior aspect is divided using sharp dissection. Identification and detachment of the cartilaginous cap of the lateral process of the malleus creates an ideal plane of dissection from superior to inferior (6). At the umbo, fibrous attachments are divided with sharp micro-scissors. All epithelium is removed from the malleus with a 45° or 90° angled hook (7) (Figure 1). The anterior malleolar ligament is identified and preserved. A meticulous removal of the epithelium seeks to avoid future iatrogenic cholesteatoma formation.
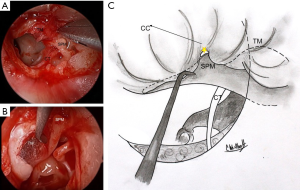
Next, endoscopic inspection of the middle ear is performed. Any epithelium migrating medial to the perforation edge should be noted and removed in order to prevent future cholesteatoma formation.
Graft harvesting and fashioning
Tragal cartilage is the graft material of choice. Procurement of this graft follows the steps described by Dornhoffer (8,9). It begins with a cut through skin, perichondrium and cartilage on the medial surface of the tragus, leaving a 1–2 mm cartilage tip in the lateral aspect of the tragus for cosmesis. The cartilage, with attached perichondrium on both anterior and posterior surfaces, is dissected with Iris scissors (8). The aim is to remove as large a piece of tragus as possible, including the free superior margin and extending inferiorly to the antihelix junction during its dissection. The thicker perichondrium attached to the anterior side of the tragal graft is peeled off, preserved and dried flat. The thinner perichondrium on the concave posterior aspect of the tragus is left attached.
Tragal cartilage holds an advantageous natural curvature that may be particularly useful during the positioning and securing of the graft. In its absence, conchal cartilage can be used as it displays a similar curvature. However, it can be brittle and more irregular, thus more difficult to fashion.
A key element in the preparation of the composite graft is to carefully remove the peripheral cartilage off the perichondrium, creating a central cartilage island with a perichondrial flange (refer to Figure 2). The firm adherence to the perichondrium ensures that the cartilage remains well positioned, a significant difference with previous techniques where the cartilage and the perichondrium are placed separately.
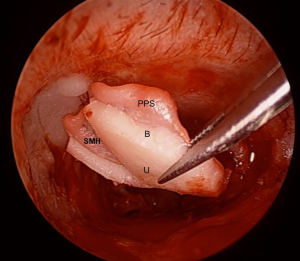
The perichondrial projection or “flange” can be placed under the perforation’s edge and line the medial aspect of the bony annulus and scutum under the TMF. In a subtotal perforation, this generally requires a large graft size of 10–12 mm (anteroposterior) by 8–10 mm (superoinferior). Generally, the size of the anterior perichondrium flange should be around 2 mm larger than the cartilage island. Superiorly the flange must be long enough to cover the exposed bony scutum, securing the grafts position. Posteriorly the flange passes over the posterior canal wall. After these areas have been covered, the TMF is repositioned. This generous extension of the perichondrial portion of the composite graft aims to provide continuity between the graft and the patient’s tissues in order to help with appropriate epithelial migration and the formation of the neotympanum.
As for the cartilaginous island of the graft, the edges of the cartilage can be beveled (45°) to provide a more natural fit. A notch for the malleus handle (2/3 of its length) is created, giving the cartilage island a narrow “u” shaped appearance. It is essential not to cut through the perichondrium when removing the cartilage (Figure 2). Peripheral cartilage is removed using a round knife and the notch created using the incudostapedial joint knife which has a smaller diameter.
Grafting
The natural concave side with attached perichondrium of the graft will face laterally, providing a better fit and a smooth continuous scaffold. In some cases, this will result in its convex surface resting in between the promontory and the medial bony sulcus of the annulus, supporting the graft and diminishing the amount of medial packing required. Cartilage rigidity facilitates placement as a one-handed maneuver in EES.
Minimal support packing is required, and generally only anteriorly (anterior meso and epitympanum). Packing involves placement of absorbable gelatin sponge. Positioning of the graft is performed in an underlay fashion relative to the perforation’s edge. The malleus handle is nestled securely within the previously created notch, with the perichondrium lying over it. Two distinct differences separate this method compared to the Dornhoffer technique (8,9). Firstly, in the Dornhoffer method the notch encompasses the entire length of the graft and secondly, the Dornhoffer graft lies medial to the malleus handle. In distinction, this method has a 2/3 notch to maintain inferior concavity of the cartilage and the graft is placed lateral to the malleus. The aim of these modifications is to secure the graft in place and provide scaffold continuity using a single handed EES method.
After repositioning of the TMF, attention is focused on the anterior angle, rearranging the graft position to correct any residual gap between graft and perforation edge. When a persistent gap is evident, free perichondrial grafts (“tuck grafts”) using excess perichondrium can be used as a gasket between the graft and tympanic membrane, a technique proposed by Marchionni et al. (Figure 3).
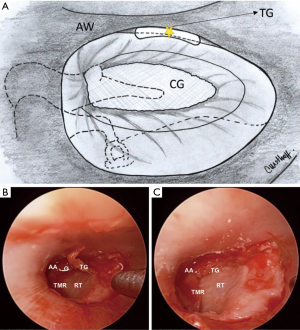
Packing with absorbable gelatin sponge over the edges of the flap/graft interface and back to the incision line is the final step.
Results
A series of 53 patients exposed to this grafting technique is presented. Mean age was 40.6 years. Twelve of these patients were pediatric (<16 years old), mean age of 11.8 years. The mean follow-up time was 9 months (range, 3–37 months). 42 (79%) cases were of primary surgeries, whilst 21% (n=11) were revision cases.
Underlying pathology in the present series was for the most part chronic suppurative otitis media (CSOM) (54%). Other associated pathologies were (in order of frequency) CSOM in conjunction with granular myringitis and/or to tympanosclerosis, perforation after an acute otitis media episode and, three perforations were traumatic in nature.
Success rate for this procedure in the present series is 85% and it is defined as absence of re-perforation throughout the follow-up time. Failures entailed 7 cases of residual pinhole perforations and one case of a large residual perforation. Of note, only one failure occurred among the revision surgery group, in association with a postoperative infection in a smoker. However, it self-healed. Figure 4 further elaborates on these data, and the posterior revision procedures performed in these cases.

Seventeen (32%) of the patients had comorbidities traditionally linked to tympanoplasty failure, including diabetes, immunosuppression and smoking. However, the procedure was successful in all but one patient who presented with an infected pinhole perforation that closed after it responded to clinical management (Figure 4).
In 14 patients, postoperative audiological data was unavailable. Audiological results were calculated based on 39 patients. Mean preoperative PTA was 42.8 dB (SD 16.7), the mean postoperative PTA was 25.7 dB (SD 15.9), with a statistically significant difference (P<0.001). Figure 4 details the change in PTA per case. The air-bone gap was reduced to 17 dB on average. Sensorineural thresholds were preserved in all patients.
No difference was found when comparing the audiological and surgical results of the adult and pediatric population.
Discussion
Composite cartilage tympanoplasty is a well-established technique. When performed endoscopically, outcomes are comparable in the short term to its microscopic counterpart (10-12), however, advantages in the visualization and access via the EES method have been noted. Cartilage is robust enough to resist middle ear negative pressure and no significant difference has been demonstrated in sound conduction when compared to temporalis fascia (8,9,13,14).
Advantages that set this method apart, involve the exploitation of the natural characteristics of the tragal cartilage, through a specific grafting design that diminishes the possibility of dislodgement and easier one-handed placement. Furthermore, this method reduces the need for middle ear packing, which reduces the likelihood of adhesion formation (15), overlying the malleus handle, offers greater scaffold uniformity and stability. This aims to prevent cartilage migration during healing, a problem which can occur when using separated cartilage and perichondrial grafts.
Anterior gaps, between the tympanic bony sulcus and the lateral surface of the neotympanum are a common problem with rigid cartilage grafts. This is addressed by the introduction of “tuck grafts” that can be more precisely placed through angled scope views.
Despite the technique’s advantages, some potential concerns exist. Early in the learning curve, dissection on the handle of the malleus may cause trauma to the ossicular chain. However, the likelihood is reduced by not placing it under the malleus and evidence suggests that SNHL is not a common outcome with moderate manipulation (16). Secondly, incomplete removal of epithelium off the handle of the malleus could lead to cholesteatoma formation. The detailed endoscopic view of the malleus provides a superior view of the plane of dissection, reducing this possibility. Thirdly, insertion of a large composite graft through the canal requires care to avoid pushing squamous epithelium into the middle ear and creating implantation cholesteatoma.
Long term outcomes for this method still require review but the present series demonstrates short term success, that the method is safe and that closure rates and hearing outcomes equal those reported using endaural and post auricular techniques. The endoscope proved very useful to achieve a transcanal approach for anterior and large perforations, as illustrated in Figure 5.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.11.03). AJS serves as an unpaid editorial board member of Australian Journal of Otolaryngology. NJ serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ayache S. Cartilaginous myringoplasty: The endoscopic transcanal procedure. Eur Arch Otorhinolaryngol 2013;270:853-60. [Crossref] [PubMed]
- Anzola JF, Nogueira JF. Endoscopic Techniques in Tympanoplasty. Otolaryngol Clin North Am 2016;49:1253-64. [Crossref] [PubMed]
- Pothier DD. Introducing endoscopic ear surgery into practice. Otolaryngol Clin North Am 2013;46:245-55. [Crossref] [PubMed]
- Glikson E, Yousovich R, Mansour J, et al. Transcanal Endoscopic Ear Surgery for Middle Ear Cholesteatoma. Otol Neurotol 2017;38:e41-5. [Crossref] [PubMed]
- El-Begermy MA, El-Begermy MM, Rabie AN, et al. Use of local anesthesia in ear surgery: technique, modifications, advantages, and limitations over 30 years’ experience. Egypt J Otolaryngol 2016;32:161-9. [Crossref]
- Michaelides E, Kansal A, Rutter S, et al. The malleus cap: Anatomic description of cartilage of the lateral process of the malleus. Am J Otolaryngol 2018;39:208-11. [Crossref] [PubMed]
- Livio P, Marchioni D. Endoscopic Ear Surgery: Principles, Indications, and Techniques. Stuttgart: Thieme; 2014.
- Dornhoffer JL. Cartilage Tympanoplasty. Otolaryngol Clin North Am 2006;39:1161-76. [Crossref] [PubMed]
- Dornhoffer J. Cartilage tympanoplasty: indications, techniques, and outcomes in a 1,000-patient series. Laryngoscope 2003;113:1844-56. [Crossref] [PubMed]
- Choi N, Noh Y, Park W, et al. Comparison of endoscopic tympanoplasty to microscopic tympanoplasty. Clin Exp Otorhinolaryngol 2017;10:44-9. [Crossref] [PubMed]
- Huang TY, Ho KY, Wang LF, et al. A comparative study of endoscopic and microscopic approach type 1 tympanoplasty for simple chronic otitis media. J Int Adv Otol 2016;12:28-31. [Crossref] [PubMed]
- Awad OG, Hamid KA. Endoscopic type 1 tympanoplasty in pediatric patients using tragal cartilage. JAMA Otolaryngol Head Neck Surg 2015;141:532-8. [Crossref] [PubMed]
- Chhapola S, Matta I. Cartilage–Perichondrium: An Ideal Graft Material? Indian J Otolaryngol Head Neck Surg 2012;64:208-13. [Crossref] [PubMed]
- Gerber MJ, Mason JC, Lambert PR. Hearing Results After Primary Cartilage Tympanoplasty. Laryngoscope 2000;110:1994-9. [Crossref] [PubMed]
- Bahadir O, Aydin S, Caylan R. The effect on the middle-ear cavity of an absorbable gelatine sponge alone and with corticosteroids. Eur Arch Otorhinolaryngol 2003;260:19-23. [PubMed]
- Kazikdas KC, Onal K, Yildirim N. Sensorineural hearing loss after ossicular manipulation and drill-generated acoustic trauma in type I tympanoplasty with and without mastoidectomy: A series of 51 cases. Ear Nose Throat J 2015;94:378-98. [PubMed]
Cite this article as: Wuesthoff C, Hardman J, Saxby AJ, Jufas N, Patel N. How I do it—endoscopic composite cartilage graft tympanoplasty. Aust J Otolaryngol 2018;1:33.


