Outcomes using the T-14 symptom score for tonsillectomy in an Australian paediatric population
Introduction
Paediatric adenotonsillectomy is a commonly performed operation by otolaryngologists around the world. The main indications for adenotonsillectomy in a paediatric population are sleep disordered breathing (SDB) and/or recurrent infections (1,2). The burden of tonsil disease has a wide-ranging impact, affecting the health system, caregivers and the patient. Poor quality of life (QoL) has been identified in children who suffer from SDB and tonsillitis compared with healthy children (3-5), and is comparable to the QoL of children who suffer from other chronic medical conditions, such as asthma (6,7) and juvenile arthritis (8).
A recent systematic scoping review (9) identified 10 generic and disease-specific questionnaires to assess the QoL outcomes of tonsillectomy, including the Paediatric Quality of Life Inventory (PedsQL), Obstructive Sleep Apnoea-18 (OSA-18) and Paediatric Throat Disorders Outcome Test (T-14). The PedsQL (10) is a generic health status questionnaire that is widely used in children aged 2–18 that gives a summary of physical functioning and psychosocial health. However, it is not specific to tonsillectomy nor its indications. The OSA-18 (8,11) is disease-specific to SDB, but does not account for recurrent infections and their associated morbidity.
These questionnaires are examples of validated instruments that rely on patient reported outcome measures (PROMs). PROMs have been increasingly utilised for assessing outcomes after surgical procedures in the field of otolaryngology (12,13). They provide an alternate perspective on the effectiveness of procedures compared with traditional surgical outcomes. Given their focus on the patient, they are a useful method of assessing QoL while allowing patients to provide feedback in the course of their care. Parental perception of their child’s symptoms and QoL can be used as a surrogate for “paediatric PROMs”.
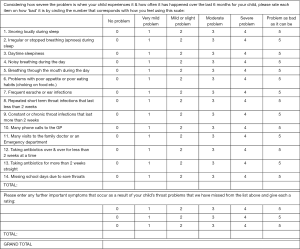
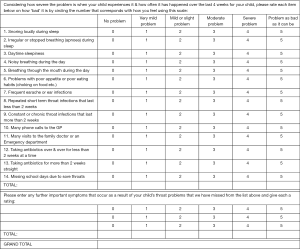
The T-14 Paediatric Throat Disorders Outcome Test is a valid, sensitive, and reliable parent-reported outcome measure for paediatric throat disorders. It was developed by a panel of surgeons from the Clinical Audit and Practice Advisory Group of ENT-UK for children with throat disorders and is applicable for children with throat disorders causing SDB or recurrent tonsillitis (14). It was based on the Tonsil and Adenoid Health Status Instrument (15). Subsequent review during the development of the T-14 reduced the item number to 14. In the final version of the T-14, 6 questions pertain to symptoms of ‘Obstruction’, while the other 8 questions are relevant to ‘Infection’.
The T-14 has been applied in numerous studies in England and Ireland (14,16-20) to demonstrate the importance of measuring patient reported outcomes with tonsillectomy, but it has not been used outside the UK. We aimed to apply the questionnaire in a paediatric Australian patient population undergoing adenotonsillectomy or tonsillectomy for SDB or recurrent tonsillitis to evaluate the effectiveness of the T-14 Paediatric Throat Disorders Outcome Test as a tool to measure health care outcomes in this group of patients.
Methods
Study design and ethical considerations
Ethics approval was provided by the Southern Adelaide Clinical Human Research Ethics Committee (Project number 123.17). A consecutive case series was performed in South Australian patients (age 1–16 years) recruited from both public and private clinics under the supervision of the senior lead author (EHO) over a period of 15 months from November 2016 to October 2017. Administration of the T-14 questionnaire to caregivers presenting with their child for consultation in the ENT clinic for their child’s throat disorder (SDB and/or recurrent tonsillitis) is a routine process. Post-operative complications such as haemorrhage are recorded as part of the ENT surgery department’s routine audit and included in this study. Routine follow up questionnaires were administered 6–8 weeks post-operatively to the caregiver presenting with their child at clinic, or via a telephone call.
T-14 questionnaire
Separate questionnaire forms were prepared for pre-surgery and post-surgery data collection (Figures 1 and 2). Minor modifications were made to the published T-14 text (14) after receiving feedback from Australian parents during a pilot study (unpublished). Question 10 was simplified from “phone calls to GP and NHS direct” to “phone calls to GP”, as NHS referred to the UK National Health Service and is not relevant in the Australian healthcare setting. Additionally, for the post-operative questionnaire, caregivers were asked to rate the symptoms within a timeframe of the last 4 weeks, instead of the last 6 months. This decision was made to provide investigators with earlier and more contemporaneous information at least 6 weeks post-operatively as their child would have recovered from their surgery. In addition to the T-14 questionnaire, extra rows allowed caregivers to input their own observations not covered by the questionnaire. For scoring, each T-14 question was attributed a score of 0–5 as in the original test (14), with 0 defined as not causing any problems up to 5 being the problem was as bad is it could be.
Statistical analysis
All patient data was de-identified using patient unit record (UR) numbers to correlate clinical information during statistical analysis. Results were collated with Microsoft ExcelTM (Microsoft Corporation, WA, USA). All patients were de-identified using a reference number and date of birth to match procedures, post-operative complications, and T-14 scores. Scores were recorded for individual questions to provide total scores and divided into section scores for ‘Obstruction’ (questions 1–6) and ‘Infection’ (questions 7–14) (14). Statistical analysis utilized non-parametric tests (IBM SPSS Statistics 22, New York, USA) with graphs prepared in Prism 7.0TM (GraphPad Software, Inc, La Jolla, CA, USA).
Results
All caregivers were able to complete a pre- and post-operative questionnaire. Of the 63 patients who were consented for this study, 45 patients had their post-surgery T-14 questionnaires administered within the 6–8 week post-operative period. The remaining 18 were administered either after this period (n=17) or earlier than 6 weeks (n=1) and therefore excluded from this study. Of the 45 patients, the median age was 6 (IQR, 3.17–8.50; range, 1.67–15.67) years. The reported indication for surgery was SDB (n=22), recurrent tonsillitis (n=12), or SDB + tonsillitis (n=11). Surgical procedures included adenotonsillectomy (n=40), tonsillectomy (n=3), subtotal adenotonsillectomy or tonsillectomy (n=2). A proportion also had grommet insertion (n=9). A variety of surgical tonsillectomy techniques were utilised including coblation (n=17), Bizact (n=14), cold steel (n=13) and monopolar (n=1). Haemorrhage occurred in 2.2% of cases (1/45) following a coblation procedure in a 3-year-old child. It occurred on post-operative day 5 and was classed as grade A2 by the Stammberger Classification Criteria (21), not requiring any intervention.
T-14 questionnaire responses were collected prior to surgery (median, 3.14 weeks; IQR, 0–8.57) reflecting the child’s health status during the previous 6 months. Post-operative questionnaire responses were obtained 6–8 weeks following surgery (median, 6.14 weeks; IQR, 6.00–7.29) reflecting the child’s health status during the previous 4 weeks (i.e., a 4-week period following a minimum of 2 weeks recovery time after surgery). T-14 total and sub-domain scores are presented in Table 1 for each presenting indication. The pre-operative Infection sub-domain scores were lower in children presenting with SDB compared to those presenting with tonsillitis or SDB + tonsillitis (KW P=0.017).
Table 1
| T-14 domain | Time | Presenting indication for surgery | |||
|---|---|---|---|---|---|
| All (n=45) | SDB (n=22) | Tonsillitis (n=12) | SDB + tonsillitis (n=11) | ||
| T-14 total score | Pre–op | 23 [13–32] | 20 [13–31] | 29.5 [17–32.5] | 29 [12–38] |
| Post–op | 3 [0–7] | 3 [1–8] | 1 [0–4.5] | 3 [1–9] | |
| Obstruction domain | Pre–op | 12 [9–16] | 13 [11–16] | 7.5 [1.5–12.5] | 13 [10–19] |
| Post–op | 2 [0–5] | 2.5 [1–5] | 0 [0–2.5] | 3 [1–7] | |
| Infection domain | Pre–op | 11 [3–20] | 3.5 [2–14] | 19 [12–22] | 14 [6–19] |
| Post–op | 0 [0–2] | 0 [0–2] | 0 [0–1] | 0 [0–2] | |
Pre-and post-operative T-14 scores, with obstruction and Infection sub-domain scores, presented as median [IQR]. SDB, sleep disordered breathing.
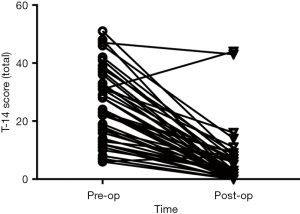
Post-operative T-14 scores were significantly decreased for the total score, as well as each sub-domain score (total, P=0.000; obstruction domain, P=0.000; infection domain, P=0.000; Related Samples Wilcoxon signed rank test; Figure 3). Analysis identified two children whose parental perception of QoL had not improved following surgery (i.e., high post-operative T-14 scores; Figure 4). For a 6-year-old that presented with SDB + tonsillitis, post-operatively the parent reported ongoing noisy breathing during the day due to nasal obstruction with allergic symptoms. A 3-month trial of intranasal corticosteroids was instituted with no improvement in symptoms. The child was subsequently scheduled for coblation channeling to their inferior turbinates +/− revision adenoidectomy. For a 2-year-old that also presented for SDB + tonsillitis, an ear infection was identified at the time of post-operative review. Antibiotic treatment was prescribed and upon subsequent follow-up the parent reported improved symptoms, however the T-14 was not repeated.

Discussion
Key findings
This study demonstrates the T-14 is applicable in an Australian paediatric population for assessing QoL following tonsillectomy for SDB and/or recurrent tonsillitis. The Australian and UK healthcare system share some similarities within the public domain, with the role of the primary care physician, universal healthcare coverage for hospital treatment and subsidised prescriptions. The T-14 is a valid questionnaire for use in tonsillectomy studies for SDB and recurrent tonsillitis and previous studies have demonstrated the internal consistency, reliability and validity of the T-14 test (14). Our study demonstrates the T-14 is applicable in an Australian paediatric population undergoing tonsillectomy.
Patient reported outcome measures (PROMs) are validated for use in several Otolaryngology conditions such as paediatric tonsillectomy, chronic sinusitis (16) and endoscopic sinus surgery (13), as there is a growing appreciation that delivery of value healthcare requires assessment and measurement of health care outcomes (22). PROMs provide evidence of the effect on patients’ health through assessment and measurement of symptoms and QoL. Questionnaires used to assess a paediatric patient’s symptoms typically use the parent as a proxy reporter. This study is the first, to our knowledge, demonstrating the symptomatic effectiveness of tonsillectomy with the T-14 in an Australian paediatric population. Parents in this study reported a significant improvement in their child’s throat symptoms post-tonsillectomy using the T-14 questionnaire. This was demonstrated in the decreased total score post-operatively regardless of the presenting indication.
Minor alterations to the T-14 were required for Australian context such as modifying question 10, which was phrased as, “Many phone calls to the GP or NHS Direct” in the UK-based T-14. NHS Direct is a nurse hotline in the UK that offers advice to patients who do not necessarily need to call the emergency hotline. There are multiple equivalent services in Australia, such as “Healthdirect Australia” and “13 HEALTH” in Queensland. For clarity to an Australian population this item was simplified to, “Many phone calls to the GP”. The difference is subtle, but the proportion of caregivers who utilised phone-based nurse triage services may not have been reflected as a result. These minor changes may have affected the T-14 consistency, but results in this study suggest that the parents using the T-14, despite the minor modifications, consistently report the benefits of tonsillectomy on a child’s symptoms.
Strengths of the study
Previous studies in the UK found that tonsillectomy had consistent benefits irrespective of the indication, age, sex or surgical technique (19). The benefit to symptomology was similar with our results, affirming the efficacy of tonsillectomy or adenotonsillectomy in an Australian paediatric population from 1 to 16 years of age. Our results also demonstrated that parents reported an improvement in their child’s T-14 scores even if their child had a post-tonsillectomy haemorrhage (from a total score of 19 pre-operatively to 1 post-operatively in this child).
In the two cases that reported an increase in the post-operative T-14 scores, one was complicated by a post-operative otitis media. This resolved after administration of antibiotics, with subjective reports from the caregiver that the patient’s symptoms had improved. The other case reported continuing symptoms of rhinitis. Despite a trial of intranasal corticosteroids, the symptoms persisted and coblation to the inferior turbinate +/− revision adenoidectomy was offered to the parents. The increase in scores in both these cases assisted the ENT surgeon in addressing other underlying symptoms and directed the need for further treatment.
Limitations in comparison with other studies
A drawback of the questionnaires was the use of caregivers as proxy responders, who do not experience the symptoms personally and are therefore at risk of recall bias. The reason for the use of parents is because children undergoing tonsillectomy are usually at an age (<5 years of age) where they lack the insight to rate or score the extent of their symptoms and the effects on their QoL. Given the role of the caregivers in caring for their symptomatic child, it remains a suitable measure of health outcomes in the paediatric population. Konieczny et al. conducted a 2-year follow up of a cohort of paediatric patients undergoing tonsillectomy demonstrating that the T-14 scores decreased after surgery and the benefit persisted at 2 years post operatively, thus indicating that parents’ perception of their symptoms and QoL was improved with surgery (20). It would appear that adenotonsillectomy or tonsillectomy has persistent long-term benefits on the child’s QoL as rated by the parent.
Clinical applications and future directions of research
Kao et al. identified several generic and disease-specific questionnaires used to assess tonsillectomy parental reported outcome measures (9). Randomised control trials (RCTs) in recent years have conventionally relied on rates of complications such as haemorrhage and pain (21,23) or objective data such as polysomnography (5,24). However, more recent RCTs use parental reported outcome measure for tonsillectomy as a primary outcome measure (25), reflecting their increasing importance in health care. Hence the importance of utilising tools such as the T-14 to assess the effectiveness of tonsillectomy in improving health care for a child’s throat disorder.
A further literature search identified only two previous Australian studies primarily utilising parental reported outcome measures in assessing the benefits of tonsillectomy. Harvey et al. used a combination of unpublished questionnaires and polysomnography to augment their study on OSA symptoms (26). Wood et al. used the Glasgow Children’s Benefit Inventory (GCBI) to compare the differences in QoL outcomes of tonsillotomy versus adenotonsillectomy (27). Of these, only the GCBI is a validated, but generic, questionnaire. There has been no disease-specific questionnaire used in any Australian studies to date.
Application of the T-14 in pre- and post-operative settings can assist the surgeon with identifying any ongoing concerns and manage appropriately. Administration of the test is low-cost and its relatively short length of time to complete eases the burden of response. Its simplicity reduces the clinician-patient knowledge gap by highlighting key symptoms to caregivers. Identifying and scoring these symptoms enables clinicians to make decisions augmented by objective data. It also provides longitudinal information about the child’s recovery with repeated T-14 testing, however this was not explored in the scope of this study. The evidence provided in this consecutive case series therefore supports the importance of symptom-based questionnaires as a measure for caregiver perception in determining the efficacy of surgery for their child.
Conclusions
Tonsillectomy remains a clinically relevant treatment option for patients who experience SDB and tonsillitis. Our results support the symptomatic benefit of tonsillectomy in improving the QoL in an Australian paediatric population. Studies utilising parental reported health outcome measures, such as the T-14 Paediatric Throat Disorders Outcome Test, are crucial in supporting contemporary indications for tonsillectomy. We have demonstrated that the T-14 is a robust tool that is simple to administer to parents and can be utilised in a wide range of clinical environments, from public hospital networks to private practices in the Australian paediatric population.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.01.02). ES serves as an unpaid editorial board member of Australian Journal of Otolaryngology. EHO serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was provided by the Southern Adelaide Clinical Human Research Ethics Committee (Project number 123.17). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baugh RF, Archer SM, Mitchell RB, et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg 2011;144:S1-30. [Crossref] [PubMed]
- Patel HH, Straight CE, Lehman EB, et al. Indications for tonsillectomy: a 10 year retrospective review. Int J Pediatr Otorhinolaryngol 2014;78:2151-5. [Crossref] [PubMed]
- Todd CA, Bareiss AK, McCoul ED, et al. Adenotonsillectomy for Obstructive Sleep Apnea and Quality of Life: Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg 2017;157:767-73. [Crossref] [PubMed]
- Burton MJ, Glasziou PP, Chong LY, et al. Tonsillectomy or adenotonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev 2014;CD001802. [PubMed]
- Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013;368:2366-76. [Crossref] [PubMed]
- Busino RS, Quraishi HA, Aguila HA, et al. The impact of adenotonsillectomy on asthma in children. Laryngoscope 2010;120:S221. [Crossref] [PubMed]
- Levin JC, Gagnon L, He X, et al. Improvement in asthma control and inflammation in children undergoing adenotonsillectomy. Pediatr Res 2014;75:403-8. [Crossref] [PubMed]
- Baldassari CM, Mitchell RB, Schubert C, et al. Pediatric obstructive sleep apnea and quality of life: a meta-analysis. Otolaryngol Head Neck Surg 2008;138:265-73. [Crossref] [PubMed]
- Kao SS, Peters MDJ, Dharmawardana N, et al. Scoping review of pediatric tonsillectomy quality of life assessment instruments. Laryngoscope 2017;127:2399-406. [Crossref] [PubMed]
- Naiboğlu B, Kulekci S, Kalaycik C, et al. Improvement in quality of life by adenotonsillectomy in children with adenotonsillar disease. Clin Otolaryngol 2010;35:383-9. [Crossref] [PubMed]
- Kobayashi R, Miyazaki S, Karaki M, et al. Evaluation of adenotonsillectomy and tonsillectomy for pediatric obstructive sleep apnea by rhinomanometry and the OSA-18 questionnaire. Acta Otolaryngol (Stockh) 2014;134:818-23. [Crossref] [PubMed]
- Alakärppä A, Alho OP. Patient-recorded outcomes and quality of life in evidence-based medicine databases on most common ear, throat and nose procedures: a systematic review. Clin Otolaryngol 2012;37:436-45. [Crossref] [PubMed]
- Prasad S, Fong E, Ooi EH. Systematic review of patient-reported outcomes after revision endoscopic sinus surgery. Am J Rhinol Allergy 2017;31:248-55. [Crossref] [PubMed]
- Hopkins C, Fairley J, Yung M, et al. The 14-item Paediatric Throat Disorders Outcome Test: a valid, sensitive, reliable, parent-reported outcome measure for paediatric throat disorders. J Laryngol Otol 2010;124:306-14. [Crossref] [PubMed]
- Stewart MG, Friedman EM, Sulek M, et al. Validation of an outcomes instrument for tonsil and adenoid disease. Arch Otolaryngol Head Neck Surg 2001;127:29-35. [Crossref] [PubMed]
- Thong G, Davies K, Murphy E, et al. Significant improvements in quality of life following paediatric tonsillectomy: a prospective cohort study. Ir J Med Sci 2017;186:419-25. [Crossref] [PubMed]
- Hardy A, Mason K, Harding L. Assessing health related quality of life before and one year after tonsillectomy, using the paediatric throat disorder outcome tool (T14). Int J Surg 2015;23:S58. [Crossref]
- Hopkins C, Almeyda R, Alreefy H, et al. Multicentre prospective clinical application of the T14 paediatric outcome tool. J Laryngol Otol 2015;129:980-5. [Crossref] [PubMed]
- Konieczny K, Biggs TC, Caldera S. Application of the Paediatric Throat Disorders Outcome Test (T-14) for tonsillectomy and adenotonsillectomy. Ann R Coll Surg Engl 2013;95:410-4. [Crossref] [PubMed]
- Konieczny KM, Biggs TC, Pringle MB. A two-year follow-up observational study of the T-14 paediatric throat disorders outcome measure in tonsillectomy and adenotonsillectomy. Ann R Coll Surg Engl 2015;97:382-5. [Crossref] [PubMed]
- Sarny S, Ossimitz G, Habermann W, et al. Hemorrhage following tonsil surgery: a multicenter prospective study. Laryngoscope 2011;121:2553-60. [Crossref] [PubMed]
- Porter ME. What is value in health care? N Engl J Med 2010;363:2477-81. [Crossref] [PubMed]
- Dadgarnia MH, Aghaei MA, Atighechi S, et al. The comparison of bleeding and pain after tonsillectomy in bipolar electrocautery vs cold dissection. Int J Pediatr Otorhinolaryngol 2016;89:38-41. [Crossref] [PubMed]
- Borgström A, Nerfeldt P, Friberg D. Adenotonsillotomy Versus Adenotonsillectomy in Pediatric Obstructive Sleep Apnea: An RCT. Pediatrics 2017;139: [Crossref] [PubMed]
- Garetz SL, Mitchell RB, Parker PD, et al. Quality of life and obstructive sleep apnea symptoms after pediatric adenotonsillectomy. Pediatrics. 2015;135:e477-86. [Crossref] [PubMed]
- Harvey JM, O'Callaghan MJ, Wales PD, et al. Six-month follow-up of children with obstructive sleep apnoea. J Paediatr Child Health 1999;35:136-9. [Crossref] [PubMed]
- Wood JM, Harris PK, Woods CM, et al. Quality of life following surgery for sleep disordered breathing: subtotal reduction adenotonsillectomy versus adenotonsillectomy in Australian children. ANZ J Surg 2011;81:340-4. [Crossref] [PubMed]
Cite this article as: Lam ME, Woods CM, Du C, Milton T, Kao SST, Huynh J, Sigston E, Ooi EH. Outcomes using the T-14 symptom score for tonsillectomy in an Australian paediatric population. Aust J Otolaryngol 2019;2:2.