Diagnosis and management of type 1 laryngeal cleft: systematic review
Introduction
Laryngeal clefts (LC) are rare congenital/embryological abnormalities involving the larynx, trachea, and oesophagus (1). Many classifications have been devised by various authors (i.e., Armitage, Evans, Pettersson), but the Benjamin and Inglis classification remains the most recognized (2-7). The diagnosis and management of type 1 LC remains controversial, with varying consensus across centres (1). This systematic review aims to provide guidance in diagnosing and providing best-practice management for this condition.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (“PRISMA”) statement was followed in this review (8). PubMed, Medline and the Cochrane library were searched from 1984 to 28 July 2018. The search was performed using the following combination of keywords: “laryngeal cleft”, “laryngotracheoesophageal cleft (LTEC)”, ”cleft larynx”, “deep interarytenoid notch”, “laryngofissure”, “diagnostic criteria”, “symptoms”, “VFSS”, “videofluoroscopic swallowing study”, “swallow study”, “radiography”, “modified barium swallow”, “flexible nasendoscopy”, “flexible laryngoscopy”, “bronchoscopy”, “esophagoscopy”, “laryngoscopy”, “microlaryngoscopy”, “laryngotracheobronchoscopy”, “suspension laryngoscopy”, “management”, “treatment”, “conservative”, “surgery”, “endoscopic repair” and “injection”.
The inclusion criteria were: case reports, case series, research survey, retrospective analyses, clinical trials and randomized controlled trials that discussed diagnosis, treatment and treatment outcomes of type 1 LC in the pediatric population. Data were of children less than 18 years of age and comprised of articles available in full text only. Treatment modalities included were medical therapy, injection augmentation and endoscopic surgery. Studies were excluded if they discussed treatment modalities not mentioned, no diagnosis or management recommendations made where appropriate, patients with congenital syndromes or complex airway anomalies such as tracheoesophageal fistula.
Two reviewers screened titles and abstracts independently according to the predefined inclusion and exclusion criteria. The full texts of potentially relevant studies were examined subsequently. Disagreements between reviewers were resolved by discussion and examination of the full text. The following information was independently extracted by the reviewers: number of patients, study design, intervention, outcome and recommendation.
A quality assessment of included studies was also undertaken by the two reviewers using the National Institutes of Health Quality Assessment Tools (NIH-QAT) for Observational Cohort and Cross-Sectional Studies and Case Series Studies (9,10).
A meta-analysis of proportions was performed for the outcome of each treatment modality, reported as improvement or resolution of symptoms. MedCalc software for Windows was used for data analysis. The pooled proportion with 95% confidence interval (CI) is given to both the fixed effects model and random effects model used in this study. The type of model used was determined by the χ2 test for heterogeneity. If a P value of <0.05 was obtained, the random effects model was used due to the presence of significant heterogeneity. The I2 statistic was used to measure the degree of heterogeneity.
Results
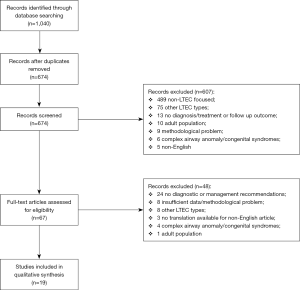
A total of 1,040 studies were obtained following a combined search of electronic databases. Six hundred and seventy-four remained after removal of duplicates, of which 67 articles were selected for full text assessment after abstract screening. The final review identified a total of 19 articles for inclusion and data extraction. The PRISMA flow diagram for article inclusion is shown in Figure 1.
Amongst the 19 articles, there were 11 retrospective reviews, 1 prospective study, 5 case series, 1 case report and 1 research survey. The characteristics of these studies are demonstrated in Table 1.
Table 1
| Study and type | Participant | Intervention | Outcome/recommendation |
|---|---|---|---|
| Berzofsky et al., 2018—retrospective cohort study | 35 | Endoscopic surgery | Patients with comorbidities affecting dysphagia had higher surgical success rates than the overall group |
| Patients with comorbidities affecting dysphagia should not be excluded from consideration for surgical repair | |||
| Dehydration and hematemesis were complications | |||
| Yeung et al., 2017—research survey/cross-sectional study | NA | NA | Feeding therapy appropriate as initial management |
| Anti-reflux therapy only when symptoms of laryngopharyngeal reflux present | |||
| 3–12 months trial of conservative therapy | |||
| Post-operative swallowing evaluation at 4–8 weeks | |||
| Boesch et al., 2017—retrospective cohort study | 96 | Flexible bronchoscopy | Flexible bronchoscopist able to identify 11/16 (68.8%) of LC in which confirmatory rigid LTB was performed |
| Wentland et al., 2016—retrospective cohort study | 31 | Endoscopic repair with post-operative MBS | Children without comorbidities—clinical evaluation at 6 weeks post-operatively and MBS at 12 weeks |
| Children with comorbidities—12 weeks post-operatively | |||
| Children with silent aspiration/laryngeal penetration—MBS at 6 weeks | |||
| Thottam et al., 2016—retrospective cohort study | 68 | Injection augmentation | Significant reduction of frank or silent aspiration |
| Long-lasting effect in some | |||
| Injection augmentation may be a more conservative option prior to surgical repair | |||
| Strychosky et al., 2016—retrospective cohort study | 175 | Pre- and post-operative MBS | MBS study helps direct feeding therapy pre- and post-operatively |
| Use of thickened feeds/repair alone may be successful if laryngeal penetration/aspiration present | |||
| If oral or pharyngeal phase impairment present, swallowing dysfunction may persist despite surgical repair | |||
| Fukumoto et al., 2015—case series | 5 | Endoscopic surgery | Laryngomalacia causing difficult extubation and laryngeal granuloma causing airway obstruction requiring repeat endoscopic surgery |
| 2.5 kg sufficient for endoscopic surgery | |||
| Alexander et al., 2015—retrospective cohort study | 54 | Endoscopic surgery | Endoscopic surgery effective and safe |
| Post-operative MBS at 8 weeks | |||
| Rossi et al., 2014—case report | 1 | Conservative management with thickened milk, anti-reflux medications and positioning techniques | Involvement of speech-language pathologist for earlier diagnosis and improved outcomes |
| Osborn et al., 2014—retrospective cohort study | 60 | Post-operative swallowing evaluation (MBS/FEES) | Post-operative MBS/FEES at 3, 6, 12 and 24 months |
| Ojha et al., 2014—retrospective cohort study | 42 | Conservative treatment with thickened feeds, anti-reflux therapy and positional techniques | At least 3 months conservative therapy |
| Surgical intervention after failed conservative management | Surgical repair should be conducted in children with comorbidities increasing aspiration risk | ||
| Post-operative MBS at 6 weeks, continue thickened feeds if no improvement | |||
| Repeat MBS at 3 monthly intervals until resolution | |||
| Referral to appropriate specialist if no sign of improvement after 6 months | |||
| Mangat et al., 2012—case series | 18 | Injection augmentation | Post-operative laryngeal swelling occurred as complication |
| Some patients do not require further intervention as they develop protective swallowing strategies | |||
| Cohen et al., 2011—case series | 10 | Injection augmentation | Endoscopic surgery if initial resolution but relapse of clinical symptoms |
| Bakthavachalam et al., 2010—retrospective cohort study | 59 | Conservative management with thickened feeds | Trial of conservative therapy up to 2 years old |
| Endoscopic surgery | Consider surgical intervention after 2 years old | ||
| Ketcham et al., 2008—case series | 16 | Endoscopic surgery | No statistically significant difference in success in patients with or without comorbidities |
| Cleft dehiscence occurred as a complication | |||
| Rahbar et al., 2006—retrospective cohort study | 2 | Conservative management with thickened feeds | Gastroesophageal reflux disease must be controlled with medical/surgical therapy prior to cleft repair |
| Surgical intervention with failed conservative therapy | |||
| Chien et al., 2006—prospective cohort study | 20 | Careful history and physical examination | All patients should receive trial of conservative prior to consideration of surgical intervention |
| Rigid LTB to confirm diagnosis | |||
| Boseley et al., 2006—case series | 3 | Conservative management with thickened feeds | Good outcomes with both conservative and surgical treatment |
| Endoscopic surgery | |||
| Watters et al., 2003—retrospective cohort study | 12 | Conservative management with anti-reflux therapy and thickened feeds | Minimum 4 months trial of conservative therapy before surgical intervention |
Eleven out of 16 (68.8%) children with type 1 LC were identified via flexible bronchoscopy (FB) in a study by Boesch et al. (11), which was performed prior to confirmatory rigid laryngotracheobronchoscopy (LTB).
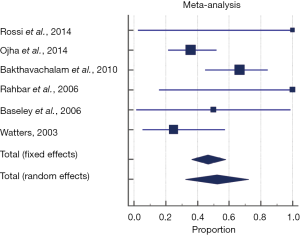
The pooled proportion of the conservative management group experiencing spontaneous symptom resolution and obtaining a normal diet for age across all included studies was 52.3% (95% CI, 32.3–71.9%). A random effects model was used to pool the proportion given significant heterogeneity seen across studies (P=0.0263, Q =12.7, I2 =60.24%) (Figure 2). Forty-one patients who failed to improve with conservative therapy went on to have surgical repair. Sixty-six point five percent (95% CI, 41.7–87.1%) were reported with “symptom resolution”, “uncomplicated” or “good outcome”. The random effects model was used to pool the proportion given significant heterogeneity seen across studies (P=0.110, Q =6.03, I2 =50.2%).
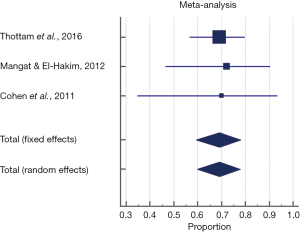
The pooled proportion of the injection augmentation group demonstrating complete resolution of either symptoms or abnormal modified barium swallow (MBS) findings was 69.2% (95% CI, 59.1–78.1%). A fixed effects model was used to pool proportion given no significant heterogeneity seen across studies (P=0.980, Q =0.041, I2 =0%) (Figure 3).
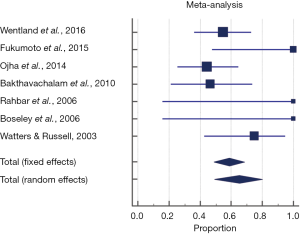
The pooled proportion of the endoscopic surgery group which were reported as successful, defined as either improvement or complete resolution of symptoms or aspiration on swallowing assessment was 75.6% (95% CI, 69.2–81.3%). A fixed effects model was used to pool proportion given no significant heterogeneity seen across studies (P=0.421, Q =9.18, I2 =1.93%). However, after excluding patients from Alexander et al. (12), Berzofsky et al. (13), and Ketcham et al.’s (14) studies which did not report outcomes of improvement and complete resolution separately, 65.4% (95% CI, 49.1–80.1%) were found to have complete resolution of symptoms or aspiration. The random effects model was used to pool proportion given significant heterogeneity seen across studies (P=0.03, Q =13.9, I2 =56.9%) (Figure 4).
Quality assessment
The NIH-QAT for Observational Cohort and Cross-Sectional Studies and Case Series Studies were used to assess the quality of included studies (9,10). Tables 2 and 3 display the individual items of quality assessment for each paper along with the overall quality assessment score.
Table 2
| Studies | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berzofsky et al., 2018 | Y | Y | Y | N | N | Y | N | NA | Y | NA | Y | N | Y | Y | Fair |
| Yeung et al., 2017—research survey | Y | Y | CD | Y | NA | NA | NA | NA | NA | NA | Y | N | NA | NA | Fair; wide involvement of experts internationally from 7 different countries, consensus was defined when majority (>80%) agreed |
| Boesch et al., 2017 | Y | Y | Y | Y | N | Y | NA | Y | Y | NA | Y | N | NA | N | Fair |
| Wentland et al., 2016 | Y | Y | Y | Y | N | Y | Y | NA | Y | N | Y | N | Y | Y | Good |
| Thottam et al., 2016 | Y | Y | Y | Y | N | Y | Y | NA | Y | N | Y | N | Y | Y | Good |
| Strychowsky et al., 2016 | Y | N | Y | N | N | Y | Y | Y | Y | N | Y | N | Y | Y | Fair |
| Alexander et al., 2015 | Y | Y | Y | Y | N | Y | CD | NA | N | NA | N | N | Y | Y | Fair |
| Osborn et al., 2014 | Y | Y | Y | Y | N | Y | Y | NA | Y | N | Y | N | Y | Y | Fair |
| Ojha et al., 2014 | Y | Y | Y | Y | N | Y | Y | NA | N | Y | Y | N | Y | Y | Fair |
| Bakthavachalam et al., 2010 | Y | Y | Y | Y | N | Y | CD | NA | N | N | N | N | Y | Y | Fair |
| Rahbar et al., 2006 | Y | Y | Y | Y | N | Y | Y | NA | N | N | N | N | Y | N | Fair |
| Chien et al., 2006 | Y | Y | Y | Y | N | Y | CD | NA | Y | N | Y | N | Y | N | Fair |
| Watters et al., 2003 | Y | Y | Y | Y | N | Y | Y | NA | Y | N | Y | N | Y | N | Fair |
Y, yes; N, no; NA, not applicable; CD, cannot determine.
Table 3
| Studies | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Fukumoto et al., 2015 | N | Y | Y | Y | Y | N | Y | NA | Y | Fair |
| Rossi et al., 2014 | Y | Y | NA | NA | Y | Y | Y | NA | Y | Fair |
| Mangat et al., 2012 | Y | Y | Y | CD | Y | Y | Y | NA | Y | Fair |
| Cohen et al., 2011 | Y | Y | Y | Y | Y | Y | CD | Y | Y | Fair |
| Ketcham et al., 2008 | Y | Y | Y | Y | Y | Y | CD | Y | Y | Good |
| Boseley et al., 2006 | Y | Y | N | Y | Y | Y | Y | NA | Y | Fair |
Y, yes; N, no; NA, not applicable; CD, cannot determine.
The NIH-QAT will allow for assessment of potential sources of bias, flaws in study methods, confounders and other relevant factors. A cumulative quality rating of “good”, “fair” or “poor” was given based on responses to the questions. Generally, a good study has the least risk of bias and is considered valid. A fair study is prone to some bias but inadequate to invalidate its findings. A poor study has high risk of bias and is considered invalid. Any discrepancies between the two reviewers were resolved via discussion.
As the studies were mostly of retrospective nature, there was a lack of standardization for follow up periods. Additionally, patient outcomes were assessed differently—some clinically, and others radiographically [MBS, functional endoscopic evaluation of swallowing (FEES)], making it difficult for comparison of outcomes. The subjective nature of a clinical assessment also predisposed to bias. Lastly, the lack of blinding in all the included studies further added on to the possibility of bias. Nevertheless, many studies attempted to clarify potential confounders (such as difference in age and presence of comorbidities), with some providing statistical analyses on their significance (12,13,15-20). The majority of studies also provided a clear description of results and outcomes.
Most of the papers were thought to be of “fair” quality except for two articles which were considered “good”. Given the undistinguished studies included, this review may not necessarily provide the most accurate assessment of evidence-based medicine with regards to managing pediatric laryngeal cleft.
Discussion
Incidence
Incidence rates have increased over the years—6.2% to 7.6% in recent years, as compared to 0.1% to 0.47% in initial reports (21-23).
Such a significant increase in incidence rates reported can be attributed to greater awareness and understanding of the disease, higher levels of suspicion, along with the availability of more advanced diagnostic tools (21,24).
Data regarding the exact epidemiology of the laryngeal cleft types remains unavailable, likely due to the rarity of laryngeal clefts. Some recent studies suggest that type 1 LC is the most commonly diagnosed (16,25,26). Other studies had varying data which indicated a more even spread amongst the different types (27,28). This variance amongst the studies could possibly be due to a particular focus on surgical management, which is the mainstay treatment of LC types 3 and 4. Additionally, there is a potentially undiagnosed population of type 1 LC because these clefts can be asymptomatic and remain clinically silent.
Comorbidities and associations
Majority of laryngeal clefts occur sporadically, although autosomal dominant traits have been observed in some families (29). Additionally, substance abuse, premature delivery, and polyhydramnios are well-recognized maternal risk factors (23,29).
Gastroesophageal reflux is a common association seen with posterior laryngeal cleft, with a reported incidence ranging from 21% to 44%. It may potentially aggravate existing pulmonary conditions and lead to failure of laryngeal cleft repair. Hence it has been advised to be evaluated and controlled prior to surgical repair. Medical therapy with acid suppression medication and/or surgical intervention may be used to manage affected cases (23,27).
Other known associated anomalies include esophageal atresia (20% to 37%) with or without tracheoesophageal fistula, tracheal or subglottic stenosis, and laryngo-tracheobronchomalacia (5,24,30,31). These anomalies can largely be excluded via an endoscopic examination (24). Neuromuscular disorders are commonly seen in patients with laryngeal clefts, with an overall incidence of 13.6% reported (32).
Additionally, genetic syndromes such as Opitz-Friaz syndrome, Pallister-Hall syndrome, DiGeorge syndrome, CHARGE syndrome, and VACTERL association are also known to be frequently associated with laryngeal clefts (33).
There are contrasting opinions regarding the impact of associated comorbidities on prognosis. de Alarcón et al. (34) demonstrated that the presence of additional airway anomalies did not impact overall success rate of laryngeal cleft surgery. Other authors noted otherwise, with significantly worse outcomes in terms of conservative and surgical success seen in patients with comorbidities conferring increased aspiration risk (15,17). A more severe clinical course has been seen in patients with concomitant laryngomalacia and type 1 LC despite laryngomalacia being a relatively benign clinical condition (35). Additional management steps in view of such associated anomalies have also been suggested, i.e., individualized postoperative pulmonary plans in those with laryngeal cleft and concomitant subglottic stenosis or laryngomalacia, and a complete evaluation of the airway during a tracheoesophageal fistula repair to exclude the presence of a laryngeal cleft (34,36).
Clinical features
Clinical manifestations of laryngeal clefts appear to correlate with the severity or type of cleft (24). Less severe clefts can be asymptomatic, and some may remain clinically silent (18,24). Some type 1 LC may also be diagnosed incidentally while undergoing an endoscopic evaluation for other reasons (19).
Mild to moderate symptoms typically manifest in type 1 LC, some of which include stridor, hoarse cries, and mild feeding difficulties (24,37). Other presentations such as aspiration, recurrent pulmonary infections and cyanosis during feeding may also occur (24,38). Additionally, respiratory consequences are usually negligible (24).
On the contrary, type 3 and 4 LCs tend to be associated with more severe pulmonary involvement, i.e., recurrent pneumonia and pulmonary mucous production (37). Type 4 LCs may also present with early onset respiratory distress requiring mechanical ventilation, which is associated with poor prognosis (24).
A review by Leboulanger et al. (24) found that common clinical symptoms of laryngeal clefts, in order of frequency are:
- Swallowing disorders, i.e., aspiration and cyanosis during feeding, chronic cough;
- Pharyngeal and laryngeal symptoms, i.e., stridor, toneless cry, pharyngeal hypersecretions;
- Respiratory symptoms, i.e., recurrent pneumonia, respiratory distress at birth.
Type 1 LCs are generally difficult to diagnose and require a high level of suspicion given the inconsistent and non-specific clinical features (38). Subsequent management is also complicated by the fact that type 1 LC can be considered for either conservative or surgical management. Therefore, it is of paramount importance to tailor treatment according to an individual’s spectrum of disease (37). Undeniably, the overall approach to diagnosing and managing type 1 LC constitutes difficult clinical decisions which this paper aims to inform and streamline.
Diagnosis
Rigid LTB
Evaluation with rigid LTB, along with palpation of the interarytenoid space remains the gold standard for diagnosing an LC (1,37,39,40). Spontaneous breathing during general anesthesia reduces the likelihood of missing tracheobronchial dyskinesia or a tracheobronchial laryngomalacia (24).
During endoscopy, the length of the LC is classified using the Benjamin and Inglis endoscopic staging system (41). Additionally, a complete assessment of the respiratory tract is recommended given the relatively high association of LCs with tracheobronchial fistulas, reportedly 10% to 60% (24).
Other pre-operative investigations such as videofluoroscopy, chest x-ray and barium swallow may potentially miss a small type 1 LC (39).
Nonetheless, it remains a challenge to distinguish a child with persistent respiratory symptoms or feeding problems secondary to other disorders, such as laryngomalacia, gastroesophageal reflux disease, birth trauma and congenital infections, from one with a laryngeal cleft. Hence, posing a difficulty of identifying the patient who requires a rigid LTB (37). Therefore, apart from potential clues such as the persistence of symptoms despite aggressive management, a high degree of suspicion is essential (37,38).
Radiological assessment chest computed tomography (CT)/chest X-rays
Routine chest X-rays are typically inconclusive, although there may be signs of parenchymal changes in association with aspiration (24). A study by Williams et al. (42) did not find any statistically significant correlation between the type of LC and the radiological changes observed on chest X-rays and CT scans.
Although radiographic imaging provides little diagnostic value, potential benefits still exist when used selectively in patients with possible associated lung abnormalities. Management was altered in 43.8% of William et al.’s (42) patient population based on additional information from CT findings. Further, MRI scans may be useful for assessing associated malformations despite the lack of diagnostic value (24).
FB
During an FB, which may be performed as a procedure in an outpatient clinic under local anesthesia, a jaw thrust maneuver is performed while the lower edge of the bronchoscope tip is used to probe the interarytenoid space (11,17). The larynx may be concurrently examined for laryngomalacia, direct aspiration, and assessed for laryngeal mobility and sensitivity (24).
However, FB alone cannot rule out presence of an LC. This is because an LC may be inadvertently overlooked due to the poor visualization of the posterior glottic space, as a result of redundant mucosa between the esophagus and trachea apposing each other and prolapsing into the cleft (23,24, 41).
A retrospective review by Boesch et al. (11) evaluated the overall diagnostic yield of FB for wheezing children. Eleven out of 16 (68.8%) children with type 1 LC were identified via FB, which was performed prior to confirmatory rigid LTB. This further emphasizes that rigid LTB remains the gold standard for diagnosis, as type 1 LC may be missed on FB despite careful evaluation by experienced bronchoscopists.
MBS & FEES
MBS (or videofluoroscopic swallowing studies) and FEES can both diagnose laryngeal penetration or aspiration (1).
MBS is a non-invasive test, albeit with inferior laryngeal anatomical visualization compared to FEES (22). Observation of deep penetration with certain dietary consistencies may predict aspiration with trial of thinner consistencies. Therefore, a more viscous consistency is introduced to the feeding regimen without further testing, to avoid additional radiation and barium aspiration (13,17). Even so, some patients with normal MBS findings were found to have respiratory symptoms that were severe enough to warrant surgical intervention, indicating that MBS findings may not correlate with symptomatology (16,43,44).
On the other hand, FEES provides direct visualization of the larynx, and allows assessment of vocal cord mobility, extent of pooling and penetration, along with localizing the site of aspiration (22). It also allows assessment of the position of spillage, i.e., lateral or anterior to posterior spillage, which may indicate an alternative cause of aspiration. Lateral spillage over the aryepiglottic folds results in suspicion of neurological aspiration, making diagnosis of laryngeal cleft less likely (17).
Boseley et al. (39) advocated the use of FEES as a diagnostic tool to establish aspiration patterns in children suspicious for type 1 LC, as well as to assist in management decisions. Although children under 1 year old can undergo FEES, there may be difficulties performing it due to the lack of cooperation and patient intolerance (22,28). Distress during the examination will affect the accuracy of the swallow assessment as crying can result in aspiration as they try to swallow simultaneously. Hence, children between the age of 3 to 12 months and over the age of 4 may be better suited for FEES (22).
The findings of MBS or FEES may be useful in guiding management. In select cases, i.e., those without comorbidities, the presence of a symptomatic cleft may be inferred when persistent interarytenoid penetration is present. Conservative management may then be initiated without the need for confirmation with LTB and its associated anesthetic risk (1). Management with thickened feeds or surgical repair of the cleft alone may be successful if laryngeal penetration/aspiration is present. However, if oral/pharyngeal phase is impaired or there is delayed triggering of swallow, persistent swallowing dysfunction may occur despite rigorous surgical repair (16).
Nonetheless, it is important to note that false negative results on MBS or FEES may occur with intermittent aspiration, and hence normal results from either test do not exclude the presence of a laryngeal cleft (22,37,45). Given the possibility of alternative diagnoses and mechanisms of aspiration, careful evaluation of MBS and FEES results is warranted prior to consideration for surgical repair (22).
Prognosis
The prognosis of LC is related to the type of cleft, associated comorbidities, and the respiratory status of the patient (24,45). Mortality rates of 42% in type 1 LC, and 46% for LCs in general have been reported in the past (45-47). In comparison to the 6% to 25% mortality rates in more recent reports, this drastic reduction in mortality can be attributed to earlier diagnosis and advancements in management (24).
Different rates of success have been reported for all forms of treatment. With conservative management, failure rates ranged from 45% to 80%, with variable length of trial (15,22,23,37,48). Failure was defined as persistence of symptoms. Regarding injection augmentation, complete resolution of symptoms was reported in 39.7% to 72% of patients (20,44,49). Surgical management seemed to indicate relatively high rates of success, ranging from 73% to 100% (12,22,23,27,48,50,51). The definition of success differed between studies, they include return to normal feeding, improvement of aspiration severity and symptom resolution (15,19,40).
Treatment
Conservative management
Conservative management encompasses management of associated comorbidities (i.e., gastroesophageal reflux disease, food allergy, eosinophilic esophagitis, reactive airway disease), as well as feeding therapy (1,6). Medical management of associated comorbidities aims to improve disorders that may contribute to the swallowing impairments. On the other hand, feeding therapy involves modification to feed thickness, feeding adjuncts, positional strategies and maneuvers to prevent aspiration (6,15,19,22,33). In a research survey by Yeung et al. (1), the majority of clinicians recommended anti-reflux therapy only in the presence of laryngopharyngeal reflux symptoms.
Patients with severe symptoms or complications of aspiration, e.g., recurrent pneumonia, may benefit from nasogastric tube feeding or gastrostomy (23,24). Nissen fundoplication should also be considered in patients with a history of gastroesophageal reflux (23,27).
The recommended duration of conservative therapy was variable amongst different authors, ranging from 1 to 12 months (1,15,22,23,37). Most patients who failed to improve with conservative therapy proceeded to have surgical intervention.
One hundred and three patients who underwent conservative therapy have been identified from seven relevant studies, 20 of whom were asymptomatic and had type 1 LC diagnosed incidentally while undergoing endoscopy for other reasons (15,19,22,23,27,33,39). Fifty-two point three percent (95% CI, 32.3–71.9%) experienced spontaneous symptom resolution, obtaining a normal diet for age, with conservative management. Forty-one patients who failed to improve with conservative therapy went on to have surgical repair. Sixty-six point five percent (95% CI, 41.7–87.1%) were reported with “symptom resolution”, “uncomplicated” or “good outcome”. It is reassuring to see modest surgical success rates despite failing a trial of conservative therapy. This suggests that conservative therapy may be appropriate as initial management (1,22).
Bakthavachalam et al. (19) have found a direct correlation between the age at diagnosis and age at resolution of aspiration in patients treated conservatively. Patients who were diagnosed after 2 years old were found to have resolution of aspiration at a significantly older age with conservative treatment. Interestingly, the age of surgical repair had no statistically significant impact on the age of resolution of aspiration until past the age of 2.
With these findings, it is recommended that patients can be managed medically up to the age of 2 years, in the absence of indications for definitive surgical repair, as mentioned later (19). Following that, surgical intervention should be considered if symptoms fail to resolve, in order to prevent permanent lung damage and its associated morbidity (23). An initial 3-month trial of conservative management with thickened feeds and positional strategies appears reasonable. Anti-reflux medication should also be administered if laryngopharyngeal reflux symptoms are present. Subsequently, a multidisciplinary team review should be conducted to determine if there is clinical improvement of symptoms. If persistent symptoms are present, there may be consideration of either endoscopic repair or injection augmentation.
Injection augmentation
Injection augmentation refers to a minimally invasive technique involving the injection of a biologic material into the interarytenoid space submucosally, increasing bulk in the region (52). It is usually consented for in conjunction with an initial endoscopy following a trial of conservative management, to avoid a second anesthetic where possible (49). As discussed previously, injection augmentation is useful in patients with concurrent comorbidities to determine if they are responsible for the patient’s symptoms.
Some clinicians may choose to conduct a trial of injection augmentation to evaluate the potential effect of a surgical repair, which is highly effective but invasive (1,44). In cases complicated by concurrent neurologic or airway anomalies, injection augmentation is useful in determining whether the laryngeal cleft or other anomalies are responsible for their symptoms (53). Benefits of injection augmentation include its short procedural time, reversibility and being less invasive than surgical repair. However, there are also associated risks such as filler migration, regional scarring, along with a possible need for repeat procedures (1).
Majority of patients, 69.2% (95% CI, 59.1–78.1%), demonstrated complete resolution of either symptoms or abnormal MBS findings. Once again, it is important to note that MBS findings do not necessarily correlate with symptom improvement (22). Injection augmentation has also been shown to provide statistically significant improvement in those with frank or silent aspiration, proven on MBS or FEES. Patients with penetration on thin liquids were found to have higher odds of successful injection augmentation. Contrastingly, those with silent aspiration had decreased odds of success. There was no difference in the odds of success for those with frank aspiration (20).
Postoperative laryngeal swelling was seen in a patient with signs of obstruction resolving within 48 hours of systemic steroids and observation (49). Injection augmentation appeared relatively safe with the remaining patients having uncomplicated procedures.
A high success rate of 90% to 100% in patients who subsequently underwent surgical repair was reported (20,44). This may possibly reflect the ability of injection augmentation to identify patients who are likely to respond favorably to formal surgical repair. Endoscopic repair is thus recommended in patients with initial resolution but with relapse of clinical symptoms (44).
The duration of symptom resolution or improvement corresponds to the half-life of the injected material (i.e., lasting days with gelfoam and months with hyaluronic acid) (49). Cohen et al. (44) observed that some patients seemed to benefit from injection augmentation for longer than expected periods. Thirty-nine out of 68 (57.4%) patients in Thottam et al.’s (20) retrospective review experienced a long-lasting effect and did not require further surgical repair. Thus, it suggests that injection augmentation may be definitive treatment in some patients, who will not require further intervention (49). A possible explanation is scarring and improved competence of the deficient interarytenoid space due to an inflammatory response produced by the injected agent (44,53). Another explanation may be the development of compensatory swallowing strategies during the period of glottal competence before the effect of the injection augmentation wears out (44,49).
Ten patients required repeat injections as the relapses coincided with the lapse of the injected material’s half-life. Of which, nine patients experienced consistent positive response. No information was provided for the remaining patient (20,49). It is important to note that all patients who underwent repeat injections in Thottam et al.’s (20) study later required formal surgical repair. This suggests that patients with relapse of symptoms are unlikely to have permanent resolution of symptoms with repeat injections, despite being a favorable candidate for surgical repair. Unfortunately, there was insufficient data to determine the success rate with a single injection. There was also no data suggesting when post-operative swallowing evaluation should be performed and after what length of time with symptom resolution, would injection augmentation be considered to have been a definitive treatment.
The follow-up period of patients who received injection augmentation ranged from 0.4 to 29 months. Two patients had unknown length of follow-up. Given the short follow-up period in some patients, perhaps if followed further, some would be seen to have return of symptoms.
Endoscopic surgery
Surgical repair of a type 1 LC was first reported in 1955 (4). Currently, there are a number of endoscopic techniques utilized in surgically managing a laryngeal cleft—including microflap approximation and suture techniques with or without laser assistance (21).
Indications for surgical repair include (1,15,21):
- Severe symptoms;
- Lack of/poor response to conservative management (i.e., medical management, feeding therapy);
- Deteriorating pulmonary function;
- Presence of other comorbidities which may contribute to aspiration risk;
- Objective findings on upper aerodigestive investigation/imaging (i.e., persistence of aspiration on MBS).
Fukumoto et al. (50) mentioned that 2.5 kg may be sufficient for endoscopic surgery as the lowest body weight was a 1-month old infant weighing 2,460 g at surgery. However, in a research survey by Yeung et al. (1), most members (65%, 13/20) did not use minimum weight as a criterion for assessing patient suitability for surgery.
Endoscopic approaches have notably high efficacy and safety for Type 1 LC repairs (54). Increased success rates were also observed with the avoidance of intubation, allowing for spontaneous breathing and enhanced exposure under general anesthesia (27).
Seventy-five point six percent (95% CI, 69.2–81.3%) of the endoscopic surgical procedures performed were successful, defined as either improvement or complete resolution of symptoms or aspiration on swallowing assessment. However, after excluding patients from Alexander et al. (12), Berzofsky et al. (13), and Ketcham et al.’s studies (14) which did not report outcomes of improvement and complete resolution separately, only 65.4% (95% CI, 49.1–80.1%) were found to have complete resolution of symptoms or aspiration. The significant failure rate may indicate that other associated anomalies may be responsible for their symptoms. This suggests that it may be worthwhile performing a trial of injection augmentation prior to definitive surgery to evaluate its potential effects. Nevertheless, Ojha et al. (15) recommend that surgical repair should still be conducted in children who have comorbidities that increase aspiration risk even though they may be unlikely to have complete resolution of symptoms. They believe that the benefit of providing some symptomatic relief outweighs the risks of surgery. Similarly, Berzofsky et al. (13) stated that such patients should not be excluded from consideration of surgical repair, with their findings of higher surgical success rates in patients with comorbidities affecting dysphagia than the overall group. Ketcham et al.’s study (14) also reported no statistically significant difference in success rates in patients with or without comorbidities.
The complications seen include a case of laryngeal granuloma causing airway obstruction, cleft dehiscence and hematemesis, two cases of laryngomalacia requiring repeat endoscopic surgery and four cases of dehydration (13,14,50).
Post-operative swallowing evaluation
There has been no consensus as to the appropriate timing for post-operative swallowing evaluation, i.e., MBS or FEES, after surgical repair of LCs (17).
MBS is recommended to be performed 6 to 8 weeks after surgery to allow time for post-operative supraglottic edema to resolve (12,15). Patients should remain on a thickened feeding regimen if they show no signs of improvement. They should then be re-evaluated at 3, 6, 12 and 24 months if swallowing problems persist (15,17,18). Majority who regained a normal diet with minor modifications did so within the first 3 months after surgery. Therefore, it is unlikely for children to recover normal swallowing after 24 months. The decision to cease swallowing evaluation in a child who persistently aspirates after cleft repair should be based on clinical judgement (18). Ojha et al. (15) have suggested that a second rigid LTB or a neurology referral should be made if studies still demonstrate aspiration at 6 months.
Based on literature review, authors proposed management pathways for otolaryngologists to differentiate type 1 LC from other otolaryngologic conditions (Figure 5). The authors recognise the limitations of these recommendations given that the available studies on the topic are of lacking quality.
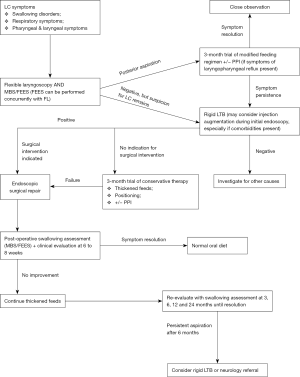
Conclusions
We recommend a diagnostic and treatment algorithm which may be considered in patients with suspected type 1 LC. All patients should have an initial 3-month trial of conservative management with thickened feeds and positional strategies. Anti-reflux therapy should be provided to patients with symptoms of laryngopharyngeal reflux. Endoscopic surgical repair should be performed in the presence of indications, i.e., severe symptoms, deteriorating pulmonary function, poor response to conservative management, persistence of aspiration on MBS or presence of other comorbidities which may contribute to aspiration risk. Prior to definitive repair, injection augmentation may be performed in selected patients with concurrent comorbidities to evaluate the potential effect of surgical repair. MBS should be performed at 6 to 8 weeks post-operatively and re-evaluated at 3, 6, 12 and 24 months if swallowing problems persist. Patients should remain on thickened feeding regimen if they show no signs of improvement. Consider repeating rigid LTB or referral to the appropriate specialist, i.e., neurologist, if there are no signs of improvement by 6 months.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.01.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yeung JC, Balakrishnan K, Cheng ATLInternational Pediatric Otolaryngoloy Group, et al. Consensus guidelines on the diagnosis and management of type I laryngeal clefts. Int J Pediatr Otorhinolaryngol 2017;101:51-6. [Crossref] [PubMed]
- Armitage EN. Laryngotracheo-oesophageal cleft. A report of three cases. Anaesthesia 1984;39:706-13. [Crossref] [PubMed]
- Evans JN. Management of the cleft larynx and tracheoesophageal clefts. Ann Otol Rhinol Laryngol 1985;94:627-30. [Crossref] [PubMed]
- Pettersson G. Inhibited separation of the larynx and the upper part of the trachea from the esophagus in a newborn: report of a case successfully operated upon. Acta Chirurgica Scandinavica 1955;110:250-4. [PubMed]
- Benjamin B, Inglis A. Minor congenital laryngeal clefts: Diagnosis and classification. Ann Otol Rhinol Laryngol 1989;98:417-20. [Crossref] [PubMed]
- Bowe SN, Hartnick CJ. Management of Type I and Type II laryngeal clefts: controversies and evidence. Curr Opin Otolaryngol Head Neck Surg 2017;25:506-13. [Crossref] [PubMed]
- Griffith CL, Liversedge TFG. Laryngeal clefts. Continuing Education in Anaesthesia, Critical Care & Pain 2015;15:237-41.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [Crossref] [PubMed]
- Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2014. (accessed 2 September 2018). Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Quality Assessment Tool for Case Series Studies. 2014. (accessed 2 September 2018). Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Boesch RP, Baughan JM, Cofer SA, et al. Trans-nasal flexible bronchoscopy in wheezing children: Diagnostic yield, impact on therapy, and prevalence of laryngeal cleft. Pediatr Pulmonol 2018;53:310-5. [Crossref] [PubMed]
- Alexander NS, Liu JZ, Bushan B, et al. Postoperative observation of children after endoscopic type 1 posterior laryngeal cleft repair. Otolaryngol Head Neck Surg 2015;152:153-8. [Crossref] [PubMed]
- Berzofsky CE, Lando T, Ettema S, et al. Indications for Surgical Repair of Type 1 Laryngeal Cleft. Ann Otol Rhinol Laryngol 2018;127:217-22. [Crossref] [PubMed]
- Ketcham AS, Smith JE, Lee FS, et al. Clinical course following endoscopic repair of type 1 laryngeal clefts. Int J Pediatr Otorhinolaryngol 2008;72:1261-7. [Crossref] [PubMed]
- Ojha S, Ashland JE, Hersh C, et al. Type 1 Laryngeal Cleft: A Multidimensional Management Algorithm. JAMA Otolaryngol Head Neck Surg 2014;140:34-40. [Crossref] [PubMed]
- Strychowsky JE, Dodrill P, Moritz E, et al. Swallowing dysfunction among patients with laryngeal cleft: More than just aspiration? Int J Pediatr Otorhinolaryngol 2016;82:38-42. [Crossref] [PubMed]
- Wentland C, Hersh C, Sally S, et al. Modified Best-Practice Algorithm to Reduce the Number of Postoperative Videofluoroscopic Swallow Studies in Patients With Type 1 Laryngeal Cleft Repair. JAMA Otolaryngol Head Neck Surg 2016;142:851-6. [Crossref] [PubMed]
- Osborn AJ, de Alarcon A, Tabangin ME, et al. Swallowing function after laryngeal cleft repair: more than just fixing the cleft. Laryngoscope 2014;124:1965-9. [Crossref] [PubMed]
- Bakthavachalam S, Schroeder JW, Holinger LD. Diagnosis and management of type I posterior laryngeal clefts. Ann Otol Rhinol Laryngol 2010;119:239-48. [Crossref] [PubMed]
- Thottam PJ, Georg M, Chi D, et al. Outcomes and predictors of surgical management in type 1 laryngeal cleft swallowing dysfunction. Laryngoscope 2016;126:2838-43. [Crossref] [PubMed]
- Aljomah D, Alshammari J. Laser Assisted Double-Layer Endoscopic Repair of Laryngeal Clefts: Our Experience in 11 Cases. Indian J Otolaryngol Head Neck Surg 2017;69:307-12. [Crossref] [PubMed]
- Chien W, Ashland J, Haver K, et al. Type 1 laryngeal cleft: Establishing a functional diagnostic and management algorithm. Int J Pediatr Otorhinolaryngol 2006;70:2073-9. [Crossref] [PubMed]
- Watters K, Russel J. Diagnosis and management of type 1 laryngeal cleft. Int J Pediatr Otorhinolaryngol 2003;67:591-6. [Crossref] [PubMed]
- Leboulanger N, Garabedian EN. Laryngo-tracheo-oesophageal clefts. Orphanet J Rare Dis 2011;6:81. [Crossref] [PubMed]
- Balakrishnan K, Cheng E, de Alarcon A, et al. Outcomes and Resource Utilization of Endoscopic Mass-Closure Technique for Laryngeal Clefts. Otolaryngol Head Neck Surg 2015;153:119-23. [Crossref] [PubMed]
- Kieran SM, Katz E, Rosen R, et al. The lipid laden macrophage index as a marker of aspiration in patients with type I and II laryngeal clefts. Int J Pediatr Otorhinolaryngol 2010;74:743-6. [Crossref] [PubMed]
- Rahbar R, Rouillon I, Roger G, et al. The presentation and management of laryngeal cleft: a 10-year experience. Arch Otolaryngol Head Neck Surg 2006;132:1335-41. [Crossref] [PubMed]
- Kubba H, Gibson D, Bailey M, et al. Techniques and outcomes of laryngeal cleft repair: an update to the Great Ormond Street Hospital series. Ann Otol Rhinol Laryngol 2005;114:309-13. [Crossref] [PubMed]
- Phelan PD, Stocks JG, Williams HE, et al. Familial occurrence of congenital laryngeal clefts. Arch Dis Child 1973;48:275-8. [Crossref] [PubMed]
- Parsons DS, Stivers FE, Giovanetto DR, et al. Type I posterior laryngeal clefts. Laryngoscope 1998;108:403-10. [Crossref] [PubMed]
- Jefferson ND, Carmel E, Cheng AT. Low inter-arytenoid height: A subclassification of type 1 laryngeal cleft diagnosis and management. Int J Pediatr Otorhinolaryngol 2015;79:31-5. [Crossref] [PubMed]
- Walker RD, Irace AL, Kenna MA, et al. Neurologic Evaluation in Children With Laryngeal Cleft. JAMA Otolaryngol Head Neck Surg 2017;143:651-5. [Crossref] [PubMed]
- Rossi MS, Buhler KE, Ventura GA, et al. Laryngeal cleft type I in neonate: case report. CoDAS 2014;26:421-4. [Crossref] [PubMed]
- de Alarcón A, Osborn AJ, Tabangin ME, et al. Laryngotracheal Cleft Repair in Children With Complex Airway Anomalies. JAMA Otolaryngol Head Neck Surg 2015;141:828-33. [Crossref] [PubMed]
- van der Doef HP, Yntema JB, van de Hoogen FJ, et al. Clinical aspects of type 1 posterior laryngeal clefts: literature review and a report of 31 patients. Laryngoscope 2007;117:859-63. [Crossref] [PubMed]
- Fraga JC, Adil EA, Kacprowicz A, et al. The association between laryngeal cleft and tracheoesophageal fistula: myth or reality? Laryngoscope 2015;125:469-74. [Crossref] [PubMed]
- Johnston DR, Watters K, Ferrari LR, et al. Laryngeal cleft: Evaluation and management. Int J Pediatr Otorhinolaryngol 2014;78:905-11. [Crossref] [PubMed]
- Slonimsky G, Carmel E, Drendel M, et al. Type I-II Laryngeal Cleft: Clinical Course and Outcome. Isr Med Assoc J 2015;17:231-3. [PubMed]
- Boseley ME, Ashland J, Hartnick CJ. The utility of the fiberoptic endoscopic evaluation of swallowing (FEES) in diagnosing and treating children with Type I laryngeal clefts. Int J Pediatr Otorhinolaryngol 2006;70:339-43. [Crossref] [PubMed]
- Thiel G, Clement WA, Kubba H. The management of laryngeal clefts. Int J Pediatr Otorhinolaryngol 2011;75:1525-8. [Crossref] [PubMed]
- Parkes WJ, Propst EJ. Advances in the diagnosis, management, and treatment of neonates with laryngeal disorders. Semin Fetal Neonatal Med 2016;21:270-6. [Crossref] [PubMed]
- Williams JL, Lee EY, Casey AM, et al. Chest radiographic and CT evaluation of lung abnormalities in pediatric patients with laryngeal cleft. Pediatr Pulmonol 2011;46:1128-33. [Crossref] [PubMed]
- Day KE, Smith NJ, Kulbersh BD. Early surgical intervention in type I laryngeal cleft. Int J Pediatr Otorhinolaryngol 2016;90:236-40. [Crossref] [PubMed]
- Cohen MS, Zhuang L, Simons JP, et al. Injection Laryngoplasty for Type 1 Laryngeal Cleft in Children. Otolaryngol Head Neck Surg 2011;144:789-93. [Crossref] [PubMed]
- Eriksen C, Zwillenberg D, Robinson N. Diagnosis and management of cleft larynx. Literature review and case report. Ann Otol Rhinol Laryngol 1990;99:703-8. [Crossref] [PubMed]
- Myer CM, Cotton RT, Holmes DK, et al. Laryngeal and laryngotracheoesophageal clefts: role of early surgical repair. Ann Otol Rhinol Laryngol 1990;99:98-104. [Crossref] [PubMed]
- Roth B, Rose KG, Benz-Bohm G, et al. Laryngo-tracheo-oesophageal cleft. Clinical features, diagnosis and therapy. Eur J Pediatr 1983;140:41-6. [Crossref] [PubMed]
- Bent JP 3rd, Bauman NM, Smith RJ. Endoscopic repair of type IA laryngeal clefts. Laryngoscope 1997;107:282-6. [Crossref] [PubMed]
- Mangat HS, El-Hakim H. Injection Augmentation of Type I Laryngeal Clefts. Otolaryngol Head Neck Surg 2012;146:764-8. [Crossref] [PubMed]
- Fukumoto K, Miyano G, Yamoto M, et al. Endoscopic repair of laryngotracheoesophageal clefts. J Pediatr Surg 2015;50:1801-4. [Crossref] [PubMed]
- Rahbar R, Chen JL, Rosen RL, et al. Endoscopic repair of laryngeal cleft type I and type II: when and why? Laryngoscope 2009;119:1797-802. [Crossref] [PubMed]
- Miglani A, Schraff S, Clarke PY, et al. An Aerodigestive Approach to Laryngeal Clefts and Dysphagia Using Injection Laryngoplasty in Young Children. Curr Gastroenterol Rep 2017;19:60. [Crossref] [PubMed]
- Kennedy CA, Heimbach M, Rimell FL. Diagnosis and determination of the clinical significance of type 1A laryngeal clefts by gelfoam injection. Ann Otol Rhinol Laryngol 2000;109:991-5. [Crossref] [PubMed]
- Koltai PJ, Morgan D, Evans JN. Endoscopic repair of supraglottic laryngeal clefts. Arch Otolaryngol Head Neck Surg 1991;117:273-8. [Crossref] [PubMed]
Cite this article as: Loh R, Phua M, Shaw CKL. Diagnosis and management of type 1 laryngeal cleft: systematic review. Aust J Otolaryngol 2019;2:5.