
Orbital exenteration: tumour diversity and survival in a tertiary referral centre
Introduction
The surgical removal of orbital contents poses a significant challenge to the patient faced with a life-threatening peri-orbital or orbital malignancy. Orbital exenteration (OE), first described by Bartisch in 1583 (1), is the surgical removal of the orbit and its surrounding adnexa and is reserved for patients who have disease not amenable to eye preserving surgical intervention (2-4). It is important the surgeon performing OE understands the impact on survival, and the cosmetic and functional challenges faced by the OE patient. Survival following OE has been investigated across a range of settings, however, varied survival statistics have been reported. Histopathological data has also been investigated in several cohorts, with diversity of tumour types and variation in tumour or pathological characteristics noted (5-14). A previous Australian cohort has demonstrated a predominance for SCC (14). Similar cohorts have been described in studies from other regions where chronic sun exposure is common (7,9). In comparison, studies from other geographical regions with lower chronic sun exposure or lower exposure to ultraviolet light more commonly have cohorts in which basal cell carcinoma (BCC) forms the dominant tumour type (6,11,12). Despite varying dominant tumour types, the clear commonality between cohorts is a degree of heterogeneity in histopathology, with cohorts reporting up to 14 different tumour types identified (6,7,9-14). As such, survival outcomes can be difficult to predict in the context of small cohorts and tumour diversity. Furthermore, Patients face significant morbidity as a consequence of OE, with risk of adverse functional and cosmetic outcomes which can impact quality of life (15). OE is a significant life event for the patient and can result in poorer health-related and vision-related quality of life (15-18). Thus, it becomes important that patients are able to make an informed decision regarding OE and are able to develop reasonable expectations regarding the likely impact surgery will have on survival and quality of life. We present demographic, tumour and survival data of patients who have undergone OE at our centre between 2005 and 2015 and discuss the results in relation to the current literature.
Methods
Prior to commencing the study, ethics approval was granted by the Human Research and Ethics Committee of the local health district. A retrospective chart review of paper based and electronic medical records, imaging and histopathology reports, and existing head and neck databases was performed. Patients were included in the study if they had undergone OE between 1st of January 2005 and 31st of December 2015, were alive at time of exenteration (i.e., exenteration was not performed for organ donation) and were aged older than 16 years at time of exenteration. Patients were excluded if they did not meet the criteria above. Data collected included epidemiological data, disease specific and histopathological data, treatment modalities, surgical data (including number of procedures performed and surgical margins), recurrence status and vitality status at the study end points.
Disease free survival (DFS) and overall survival (OS)
DFS and OS was determined following review of existing databases, medical records and through liaison with the patients’ general practitioner and treating specialists. Kaplan Meier analysis was performed to estimate DFS and OS using IBM SPSS Statistics (Version 25) software and Kaplan Meier curves were generated. Subgroup analysis was performed of tumour subgroups to generate survival curves, and Log Rank analysis (Mantel Cox) was performed to determine significance between subgroups.
Results
Cohort data
A total of 35 orbits of 35 patients were included for statistical analysis (Table 1). There were 23 male and 12 female patients (male:female of 1.9:1). The median age at exenteration was 69.4 years old (range 32.1 to 91.4 years). Patients who underwent OE for squamous cell carcinoma (SCC) were older (median age 84.6 years) compared to those who underwent OE for BCC (68.7 years) or other tumours (59.1 years). Ten of 35 patients gave a history of smoking (28.6%), and all but two patients were independent in activities of daily living prior to exenteration. Exenteration was performed for recurrent tumours in 12 patients (28.6%): 7 in the SCC group, 3 in the BCC group and 2 in the other tumour types group (1 neuroblastoma and 1 melanoma). All patients except for two (one who died days after surgery, and the other was a palliative procedure in the setting of metastatic melanoma) received adjuvant radiotherapy. Additionally, 9 patients (25.7%) required secondary surgery following exenteration, with indications ranging from treatment of recurrence, to staged reconstruction and flap revision, to debridement and management of post-operative wound infection.
Table 1
| No. | Histology | Subsites | Location | Performed in the setting of recurrence | Additional surgery | Clear margins | PER or LYM invasion | Recurrence (months) | Status at last follow up | Smoker | Sex |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Neuroblastoma | Nil | Nasal/Ethmoid | Yes | No | Yes | LYM | Yes [9] | Deceased [12] | No | Male |
| 2 | Melanoma | Thoracic; hepatic | Choroid | Yes (palliative) | No | Yes | Nil | Metastatic at time of surgery | Deceased [5] | No | Male |
| 3 | Adenoid cystic carcinoma | Nil | Nasolacrimal | No | Yes (recurrence) | No | Nil | Yes [24] | Alive | No | Female |
| 4 | SCC | Parotid | Upper lid | No | No | Yes | Both | No | Deceased [13] (upper gastrointestinal bleed) | No | Male |
| 5 | Sarcoma | Nil | Posteromedial orbit | No | Yes (stage II OST) | Yes | PER | No | Alive | No | Male |
| 6 | RHA | Nil | Alveolar | NO | No | Yes | Nil | No | Alive | Yes | Male |
| 7 | SCC | Nil | Lateral canthus | No | No | Yes | Nil | No | Alive | Unclear | Male |
| 8 | BCC | Nil | Medial canthus | Unclear | Unclear | No | Nil | No | Lost to follow up | No | Female |
| 9 | SCC | Nil | Ethmoid | Yes | No | No | Nil | No | Lost to follow up | No | Female |
| 10 | BCC | Nil | Orbit/Peri-orbit | No | No | No | Nil | No | Deceased [34] | Yes | Male |
| 11 | Adenoid cystic carcinoma | Nil | Maxillary sinus | No | No | No | PER | No | Alive | Yes | Male |
| 12 | SCC | Nil | Ethmoid | Yes | No | No | LYM | No | Deceased [76] | No | Male |
| 13 | Sinonasal undifferentiated carcinoma | Nil | Maxilla | No | No | No | Nil | No | Deceased [26] | No | Female |
| 14 | SCC | Nil | Sinonasal | No | No | Yes | Nil | No | Alive | No | Male |
| 15 | BCC | Nil | Lower lid | Yes | No | No | Nil | No | Alive | Yes | Male |
| 16 | SCC | Nil | Upper lid | Yes | Yes | Yes | Nil | No | Alive | No | Female |
| 17 | SCC | Nil | Cheek | No | No | Yes | Nil | No | Alive | Yes | Male |
| 18 | SCC | Nil | Conjunctiva | Yes | No | Yes | Nil | Yes [22] | Deceased [34] | Yes | Male |
| 19 | Oncocytic carcinoma | Nil | Nasolacrimal/Orbit | Unclear | Yes | No | Both | Yes [5] | Deceased [41] | Unclear | Male |
| 20 | SCC | Nil | Maxillary sinus/lacrimal | Yes | No | Yes | Nil | No | Deceased [83] (Pneumonia) | No | Female |
| 21 | Adenoid cystic carcinoma | Nil | Nasolacrimal | No | Yes | Yes | Nil | No | Alive | No | Female |
| 22 | Transitional cell carcinoma | Nil | Nasal | No | No | Yes | Nil | No | Alive | No | Female |
| 23 | BCC | Nil | Medial canthus | No | No | Yes | Nil | No | Alive | Yes | Male |
| 24 | SCC | Nil | Orbit | Yes | No | Yes | Nil | No | Alive | No | Male |
| 25 | SCC | Nil | Nasal | No | No | Yes | Nil | No | Alive | No | Male |
| 26 | SCC | Nil | Lateral canthus | No | No | Yes | Nil | No | Deceased [9] | No | Female |
| 27 | Melanoma | Nil | Nasal | No | No | Yes | Nil | Yes [60] | Deceased [64] | No | Male |
| 28 | SCC | Nil | Cheek | No | Yes | Yes | Nil | No | Alive | No | Male |
| 29 | SCC | Nil | Cheek | No | No | Yes | LYM | No | Alive | Yes | Female |
| 30 | Sinonasal undifferentiated carcinoma | Nil | Lacrimal | No | Yes | Yes | Nil | No | Alive | No | Female |
| 31 | SInonasal undifferentiated carcinoma | Nil | Sinonasal | No | No | No | Nil | Yes [3] | Deceased [63] | Yes | Male |
| 32 | BCC | Nil | Nasal | Yes | No | Yes | PER | No | Alive | No | Female |
| 33 | Small cell neuroendocrine carcinoma | Nil | Ethmoid | No | Yes (EVD insertion) | No | Nil | No | Deceased (3 days) | Yes | Male |
| 34 | BCC | Nil | Cheek | Yes | No | Yes | Nil | Yes [51] | Alive | No | Male |
| 35 | SCC | Nil | Medial canthus | Yes | Yes | No | Nil | No | Deceased [7] (ischaemic colitis and sepsis) | No | Male |
Patient cohort demonstrating cohort tumour diversity. SCC, squamous cell carcinoma; RHA, rhabdomyosarcoma; BCC, basal cell carcinoma; OST, osteointegration; EVD, external ventricular drain; PER, perineural; LYM, lymphovascular.
Histopathological diagnosis
There were ten different tumour types identified. Of note, 42.9% of the cohort who underwent OE had a confirmed diagnosis of SCC (15 patients). There were 6 patients with confirmed BCC (17.1%) and 14 patients who underwent OE for other tumour types: 3 adenoid cystic carcinoma, 2 sarcoma (1 rhabdomyosarcoma), 3 sinonasal undifferentiated carcinoma, 2 melanoma, 1 neuroblastoma, 1 small cell neuroendocrine carcinoma, 1 oncocytic carcinoma and 1 transitional cell carcinoma.
Surgical margins
Twelve patients (34.3%) had positive surgical margins and all but one (who died shortly after surgery) underwent adjuvant radiotherapy or chemo-radiotherapy. Three of these patients went on to develop recurrence (25%), three died from causes not related to their disease (one patient developed an intracranial haemorrhage shortly after surgery, one died 7 months after surgery from ischaemic colitis and sepsis, the third died from ischaemic heart disease), and four remained disease free during the study period. DFS of patients with involved surgical margins was 44.96 months (25.25 to 64.66 months), and OS was 54.67 months (36.05 to 73.29 months).
Perineural and lymphovascular invasion
On histopathological analysis, 5 patients (14.3%) exhibited perineural spread (2 with associated lymphovascular invasion) and 3 patients (8.6%) had lymphovascular invasion alone.
DFS
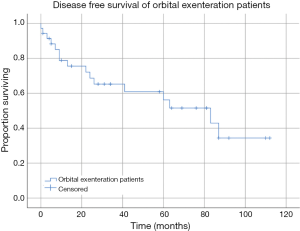
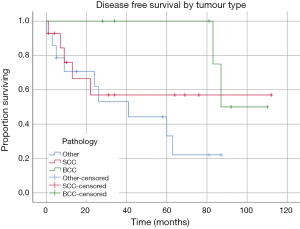
Seven patients had a confirmed recurrence of their disease. Of these, only one had involved surgical margins, all received radiotherapy or chemo-radiotherapy, and three patients had undergone OE in the setting of recurrence following prior surgical intervention of their tumour. Histopathology of the tumours that recurred included one patient with SCC, one with adenoid cystic carcinoma, one patient with neuroblastoma, one melanoma, one BCC and one undifferentiated carcinoma. Kaplan-Meier estimates of DFS revealed mean DFS of the cohort was 64.57 months (95% CI: 48.45 to 80.71 months) (Figure 1). Mean DFS of the tumour subgroups was 68.56 months (95% CI: 39.39 to 97.74 months) for the SCC group, 97.50 months (95% CI: 85.17 to 109.83 months) in the BCC group, and 42.14 months (95% CI: 24.47 to 59.80 months) in the other tumour group (Figure 2). There was no statistically significant difference between tumour subgroups (P=0.099).
OS
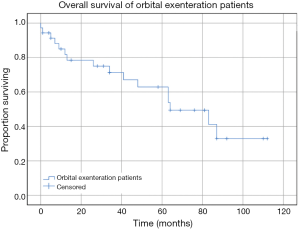
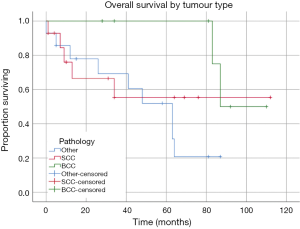
Kaplan Meier estimates of OS revealed mean survival for the cohort was 66.81 months (95% CI: 51.56 to 82.06 months) (Figure 3). This included all causes of death. Patients who had a confirmed SCC requiring exenteration had a mean OS of 68.46 months (95% CI: 39.35 to 97.59 months), in comparison the 97.50 months (95% CI: 85.17 to 109.83 months) for the BCC group and 49.07 months (95% CI: 33.11 to 65.02 months) for the other tumour group (Figure 4). There was no statistically significant difference between survival for tumour subgroups (P=0.131).
Discussion
OE is a highly invasive surgical procedure with cosmetic and functional consequences that can adversely impact quality of life. In the setting of malignancy, exenteration is performed for patients who have life threatening disease not amenable to less invasive surgical procedures.
Survival
There have been several studies investigating the survival of OE patients across a range of centres. In our cohort, we report DFS of 64.57 months and OS of 66.81 months, which is comparable to other similar size studies and similar cohorts. Aryasit et al. reported OS of 46.68 months (3.89 years) and event free survival (EFS) of 36.12 months (3.01 years) in 39 patients who underwent OE for malignancy (9). Hoffman et al in a study of 31 consecutive patients undergoing OE over a period of 7 years reported OS of 78.4 months (14). Other studies have reported 48–67% three-year survival and 37–68.3% survival at 5 years (7,12,13). Wu et al., in a study of 24 patients who underwent OE in the setting of invasive maxillary sinus SCC reported 5-year survival 27.3% (19). Ten-year survival of 37% was reported in one study (12). DFS is equally diverse in the literature. Gerring et al. reported DFS in a cohort of 49 patients who underwent OE for BCC, SCC and sebaceous gland carcinoma (DFS 52.6, 39.2 and 28.1 months respectively) (8). Roche et al. reported recurrence of 36% in 22 patients (20). Our recurrence rate in comparison was 25.7%.
Tumour histology
The tumour histopathology of patients undergoing OE has historically been diverse. In our cohort, we divided our patients into three subgroups: SCC, BCC and other tumour types based on the frequency of tumour types. There were in total 10 different tumour types. SCC was the most common (15 patients) followed by BCC (6 patients). The tumour histopathology in our cohort is similar to the numbers reported by Hoffman et al. who reported 15 cases of SCC, 8 BCC and 8 other tumour types in a cohort of 31 patients undergoing OE in a similar setting (14). Mouriaux et al. (7) and Aryasit et al. (9) also published a similar case mix to ours. Ben Simon et al. in a cohort of 34 patients reported SCC as the dominant tumour type, followed by BCC and melanoma (21). The authors suggested that a predominance of SCC in their cohort may be attributed to high index of suspicion for BCCs and early intervention. Other centres have reported higher rates of BCC than SCC in their patient cohorts. Rahman et al. 2005 reported 28 cases of BCC leading to OE in a cohort of 68 (compared with 6 cases of SCC) (6) and Wong et al. in a cohort of 73 patients reported 27 BCC and 14 SCC (11). Similarly, Rathbun et al. described 14 cases of BCC in a cohort of 48, and 6 cases of BCC (22), and Nassab et al. reported 17 cases of BCC in 32 patients undergoing OE (4). The most consistent feature amongst cohorts is significant tumour histological diversity, with studies reporting up to 14 tumour types described as indications for OE (9). Our cohort supports the complexity of tumour histopathology in patients undergoing OE.
Perineural and lymphovascular invasion
Perineural invasion (PNI) is considered an important prognostic factor for malignancies involving the orbit. Peri-orbital SCC in particular, has demonstrated PNI in a significant proportion of patients undergoing surgical resection, with time to treatment an important factor for reducing orbital invasion and PNI (23). In patients with SCC, PNI and lymphovascular invasion are both associated with increased risk of metastases and death from disease (24). Adenoid cystic carcinoma is also associated with a particularly poor prognosis due to its protracted course, and high rates of PNI (25). In our cohort 5 patients exhibited PNI with or without lymphovascular invasion: adenoid cystic carcinoma (1 of 3 patients with this pathology), SCC, BCC, sarcoma and oncocytic carcinoma. Subgroup survival analysis was not performed in the setting of small numbers of patients with PNI and lymphovascular invasion.
Involved surgical margins
Involvement of surgical margins in our cohort was demonstrated in 12 of 35 patients (34.8%). This is in keeping with other cohorts which report involved margins in 22–52% of patients (8,12,13). The impact of involved surgical margins has been evaluated in a range of studies in the literature. The current literature demonstrates that involved surgical margins do not appear to impact OS but are associated with increased risk of local recurrence. In our cohort, of those with involved surgical margins, 25% had local recurrence of their disease, and those with recurrence had a lower DFS and OS relative to the rest of the cohort. Rahman et al. (12) found there was no statistically significant difference in 3-year survival (P=0.99) in patients with involved versus clear surgical margins in a cohort of 64 patients (34 patients with clear margins and 30 with involved margins). Additionally, they demonstrated that patients with clear surgical margins had a 5-year survival of 53% compared with 63% in the involved surgical margins which was not statistically significant (P=0.454). Subgroup analysis did not show any statistically significant survival differences when assessing surgical margins in tumour subgroups in their study (12). Similarly, Nagendran et al. (13) showed no difference in survival in clear versus involved margins (P=0.12). Aryasit et al. (9) analysed the survival of patients in a cohort of 39, comparing those with involved surgical margins with those who were clear. There was no significant difference in OS demonstrated in this cohort (P=0.597), however the authors noted that a high proportion of patients within the group who had involved surgical margins also had high rates of nodal or distant metastases at time of diagnosis. Similarly, Mouriaux et al. (7) demonstrated no significant difference in OS in patients with involved versus clear surgical margins (P=0.13). They did demonstrate, however, that clear surgical margins significantly improved rates of local recurrence (P=0.01). In comparison, Gerring et al. (8) demonstrated involved surgical margins were associated with poorer prognosis in patients with non-melanoma skin cancers who underwent OE. Of 49 patients studied in this cohort, 11 patients (5 BCC and 6 SCC) had positive surgical margins. Multivariate analysis demonstrated that involved surgical margins were a predictor of poorer prognosis (P=0.002, 95% CI: 2.73–102.11. The authors identify that the results may be attributed to sample size, and comparisons may be difficult due to heterogeneity of inclusion criteria across studies.
Conclusions
OE is reserved for patients with life threatening peri-orbital or orbital malignancy for whom less invasive surgical procedures would not be adequate. We report our data of patients who have undergone OE at our centre, with reasonable survival outcomes. The tumour histology in our cohort is diverse, which is consistent with the literature. The significant morbidity associated with OE, and risk of recurrence following surgery highlights the importance of informed consent and discussion about likely survival and quality of life following surgery.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.09.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Prior to commencing the study, ethics approval was granted by the Human Research and Ethics Committee of the local health district. Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bartisch G. Ophthalmodoulcia. Dresden 1583;3:208.
- Sagili S, Malhotra R. Orbital exenteration: indications, techniques and complications. Expert Rev Ophthalmol 2016;11:201-13. [Crossref]
- Perman KI, Baylis HI. Evisceration, enucleation, and exenteration. Otolaryngol Clin North Am 1988;21:171-82. [PubMed]
- Nassab RS, Thomas SS, Murray D. Orbital exenteration for advanced periorbital skin cancers: 20 years experience. J Plast Reconstr Aesthet Surg 2007;60:1103-9. [Crossref] [PubMed]
- Bartley GB, Garrity JA, Waller RR, et al. Orbital Exenteration at the Mayo Clinic. Ophthalmology 1989;96:468-73. [Crossref] [PubMed]
- Rahman I, Cook A, Leatherbarrow B. Orbital exenteration: a 13 year Manchester experience. Br J Ophthalmol 2005;89:1335-340. [Crossref] [PubMed]
- Mouriaux F, Martinot V, Pellerin P, et al. Survival after malignant tumors of the orbit and periorbit treated by exenteration. Acta Ophthalmol Scand 1999;77:326-30. [Crossref] [PubMed]
- Gerring RC, Ott CT, Curry JM, et al. Orbital exenteration for advanced periorbital non-melanoma skin cancer: prognostic factors and survival. Eye (Lond) 2017;31:379-88. [Crossref] [PubMed]
- Aryasit O, Preechawai P, Hirunpat C, et al. Factors related to survival outcomes following orbital exenteration: a retrospective, comparative, case series. BMC Ophthalmol 2018;18:186. [Crossref] [PubMed]
- Levin PS, Dutton JJ. A 20-year series of orbital exenteration. Am J Ophthalmol 1991;112:496-501. [Crossref] [PubMed]
- Wong JC, Thampy R, Cook A. Life expectancy following orbital exenteration. Br J Ophthalmol 2015;99:1-4. [Crossref] [PubMed]
- Rahman I. Mortality following exenteration for malignant tumours of the orbit. Br J Ophthalmol 2005;89:1445-8. [Crossref] [PubMed]
- Nagendran ST, Lee NG, Fay A, et al. Orbital exenteration: The 10-year Massachusetts Eye and Ear Infirmary experience. Orbit 2016;35:199-206. [Crossref] [PubMed]
- Hoffman GR, Jefferson ND, Reid CB, et al. Orbital Exenteration to Manage Infiltrative Sinonasal, Orbital Adnexal, and Cutaneous Malignancies Provides Acceptable Survival Outcomes: An Institutional Review, Literature Review, and Meta-Analysis. J Oral Maxillofac Surg 2016;74:631-43. [Crossref] [PubMed]
- Rasmussen ML, Ekholm O, Prause JU, et al. Quality of life of eye amputated patients. Acta Ophthalmol 2012;90:435-40. [Crossref] [PubMed]
- Ye J, Lou L, Jin K, et al. Vision-Related Quality of Life and Appearance Concerns Are Associated with Anxiety and Depression after Eye Enucleation: A Cross-Sectional Study. PLoS One 2015;10:e0136460. [Crossref] [PubMed]
- Kondo T, Tillman W, Schwartz T, et al. Health-Related Quality of Life After Surgical Removal of an Eye. Ophthalmic Plast Reconstr Surg 2013;29:51-6. [Crossref] [PubMed]
- Amaro TA, Yazigi L, Erwenne C. Depression and quality of life during treatment of ocular bulb removal in individuals with uveal melanoma. Eur J Cancer Care (Engl) 2010;19:476-81. [Crossref] [PubMed]
- Wu X, Tang P, Qi Y. Management of the orbital contents in radical surgery for squamous cell carcinoma of the maxillary sinus. Chin Med J (Engl) 1995;108:123-5. [PubMed]
- Roche P, Timon C. Orbital exenteration in periorbital malignancies. The Surgeon 2012;10:189-93. [Crossref] [PubMed]
- Ben Simon GJ, Schwarcz RM, Douglas R, et al. Orbital exenteration: One size does not fit all. Am J Ophthalmol 2005;139:11-7. [Crossref] [PubMed]
- Rathbun JE, Beard C, Quickert MH. Evaluation of 48 cases of orbital exenteration. Am J Ophthalmol 1971;72:191-9. [Crossref] [PubMed]
- Soysal HG, Markoç F. Invasive squamous cell carcinoma of the eyelids and periorbital region. Br J Ophthalmol 2007;91:325-9. [Crossref] [PubMed]
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of Primary Cutaneous Squamous Cell Carcinoma With Perineural Invasion. JAMA Dermatol 2013;149:35-41. [Crossref] [PubMed]
- Fordice J, Kershaw C, El-Naggar A, et al. Adenoid Cystic Carcinoma of the Head and Neck. Arch Otolaryngol Head Neck Surg 1999;125:149. [Crossref] [PubMed]
Cite this article as: Cumming B, Sideris A, Holmes TR, Jacobson I, Havas T. Orbital exenteration: tumour diversity and survival in a tertiary referral centre. Aust J Otolaryngol 2019;2:23.


