
Evaluating and comparing the efficacy of intratympanic high dose dexamethasone (24 mg/mL) and high dose methylprednisolone (125 mg/mL) as a primary and salvage treatment for idiopathic sudden sensorineural hearing loss
Introduction
Sudden sensorineural hearing loss (SSNHL) is condition whereby the hearing loss (HL) is abrupt, sensorineural in nature, of a value greater than 30 dB in three consecutive frequencies on pure tone audiometry (PTA) and persisting over more than 72 hours. Associated symptoms often include of tinnitus, aural fullness or blockage, and dizziness (1). Of all the reported cases of SSNHL, 90% are thought to be idiopathic and this condition has been found to have an annual incidence rate of 5 to 20 people per 100,000 among the Australian adult population (2).
The diagnosis of ISSNHL can only be made when no cause could be identified following a thorough history, clinical examination, laboratory and imaging studies (2,3). Although the exact mechanism is not known, Eisenman et al. have suggested that idiopathic SSNHL may not be a simple disease entity, but that it could in fact be the clinical result of multiple conditions including; circulatory disorders, viral infection, labyrinthine membrane rupture, and autoimmune reactions (4,5). As the aetiopathophysiology of ISSNHL appears to be multifactorial in nature, many treatment regimens have since been implemented (4). Of these various treatment modalities, the use of corticosteroids (as a monotherapy or in combination with other drugs) have been proven most effective for the treatment of ISSNHL (3,6). In fact although spontaneous recovery has been reported in 32–65% within the first 3 weeks of onset (6-8), recent studies have shown that steroid therapy, if started early, may further increase recovery rates to 49–85% (6,7). The use of steroids hence remains the mainstay of treatment for ISSNHL.
Corticosteroids can be either delivered systemically (oral or intravenous) or locally into the middle ear cavity via the intratympanic route. Systemic high dose corticosteroid therapy (SCT) was traditionally used in the management of ISSNHL, however its long-term use is not encouraged because of numerous and significant side effects (6,9,10). Intratympanic steroids (ITS) therapy has hence been increasingly implemented due to its better side effect profile. ITS are directly absorbed through the round window of the cochlear, into the perilymph via pinocytosis (9,10), therefore minimising systemic side effects. Only minor side effects have been reported with ITS and all of which were transient or easily treatable (9). ITS therapy also allows a higher dose of steroids to be delivered into the perilymph (9,10), since uptake of steroids by auditory hair cells after ITS therapy is similar to that of high dose SCT (11).
The three principal use of IT steroids that have been suggested are: (I) as an initial therapy for ISSNHL without systemic therapy, (II) as an initial therapy for ISSNHL with concomitant use of systemic steroid therapy, and (III) as a salvage therapy for ISSNHL which failed to improve hearing after initial systemic therapy (3). Poor response to systemic therapy is observed in around 30–50% of patients (6). The use of ITS therapy is hence especially indicated in patients in whom SCT is contraindicated or in those with poor response (6).
Currently, there is no standardised protocol regarding the dose, frequency, method of delivery or duration of treatment in order to achieve optimal results as the use of ITS still remains controversial (3). To date, several methods of delivery have been implemented and these include direct injection into the middle-ear space via myringotomy, administration through ventilation tubes, and use of a round window transport facilitator such as a microwick (3,12). Dexamethasone and Methylprednisolone are the two main steroids that have been used in ITS therapy in the treatment of ISSNHL. The choice of steroid remains controversial, however it has recently been postulated that dexamethasone may be more efficacious as it is absorbed more readily into the endolymph (10). A 2015 meta-analysis (13) by Ng et al., also concluded that dexamethasone was preferred over methylprednisolone and that injection of steroids into the middle ear via ventilation tubes, was superior to using a microcatheter.
In 2013, the Department of Otolaryngology Head and Neck Surgery of Modbury Hospital, Adelaide, South Australia, conducted a prospective pilot study observing the efficacy of a 4-week course of low-dose intratympanic dexamethasone (ITD) therapy (8 mg/mL) as a salvage treatment for patients with ISSNHL who had failed systemic therapy (3). Results from this study failed to demonstrate any improvement in hearing on PTA with the use of low dose dexamethasone as part of a salvage therapy.
Since higher the concentrations of corticosteroids is expected to yield better outcomes (14), the aim of our study is to conduct a similar pilot study with high dose dexamethasone as well as with methylprednisolone of equal potency.
Methods
The Preferred Reporting Items for Observational studies ‘STROBE’ (Strengthening the Reporting of Observational Studies in Epidemiology) statement checklist (15) was followed in this article.
Subjects
The pilot study was carried out in the Modbury Hospital, Adelaide, South Australia. This study only included patients presenting with (I) sudden HL of 30 dB or more over three contiguous audiometric frequencies, which occurred within 72 hours, and (II) for which no cause for the sudden loss of hearing was successfully identified on thorough history, clinical examination, and magnetic resonance imaging (MRI) scan. Furthermore, informed consent was obtained from all patients and all the collected data was de-identified. Patients with associated tinnitus and vertigo at presentation were also included. There were no age restrictions for this study.
All the participants were thoroughly informed and consented prior to taking part in the study. Of note, this study does not require formal ethics approval as intratympanic steroid as a primary or salvage treatment for ISSNHL has been widely studied and is currently considered to be an effective treatment by many international institutions.
The first cohort of a total of twenty patients presented to the Otolaryngology Department of Modbury Hospital between January 2013 and December 2015 and were treated with high dose ITD (24 mg/mL). Seven received primary ITD as they could not tolerate systemic prednisolone therapy. The remainder thirteen patients received high dose ITD as part of a salvage therapy, following failure of a 3 weeks course of tapering oral prednisolone, starting at 1 mg/kg and with a maximal dose of 50 mg/day. The inability of showing an improvement of more than 10 dB on PTA at the end of the oral prednisolone course, indicates failure of oral therapy. None of the patients showed a significant improvement in hearing at the end of the high dose oral prednisolone course and were hence all included in the ITS study as part of a salvage therapy. The desired high dose Dexamethasone concentration of 24 mg/mL was directly prepared and stocked by the hospital pharmacy and made available for the purpose of the study. High dose Dexamethasone (24 mg/mL) was however no longer available after December 2015.
The second cohort of a total of ten patients presented to the Otolaryngology Department of Modbury Hospital between January 2016 and June 2018 and were treated with high dose Methylprednisolone (125 mg/mL) (ITM) which has equivalent potency as high dose Dexamethasone (24 mg/mL). The desired concentration of high dose methylprednisolone was prepared by the surgeons in the theatre or in the outpatient department prior to the procedure, as methylprednisolone was readily available in a powder form. This methylprednisolone concentration of 125 mg/mL was obtained by mixing 500 mg of methylprednisolone powder (in a vial) with 4 mL of sterile water. 1 mL of this mixture has 125 mg of methylprednisolone, which has equivalent potency as 1 mL of 24 mg/mL of dexamethasone. The same exclusion/inclusion criteria was used for the Methylprednisolone (125 mg/mL) group. Out of the ten participants, nine received intratympanic therapy as part of a salvage therapy following failure of recovery after receiving a 3 weeks course of oral prednisolone. Similarly, no significant improvement had been observed in HL at the end of the high dose oral prednisolone course.
Technique
The first dose of 1 mL of high dose dexamethasone or methylprednisolone was injected slowly into the middle ear through ventilating tube under general anaesthesia in the posteroinferior quadrant of the tympanic membrane of the affected ear of all patients. The procedure was done under general anaesthesia as a means to both standardising the technique of insertion, especially in patients with small narrow ear canals, but also to reduce the levels of discomfort and anxiety in some patients. The insertion of the tubes was either performed within 4 weeks of completion of systemic oral prednisolone treatment (in patients who were receiving ITS as part of a salvage therapy) or within the first week of presentation in patients who received ITS as a primary treatment. The delay in presentation for patients receiving ITS as a primary treatment ranged from 7 to 20 weeks after onset of ISSNHL.
The patient was kept in the lateral position with the affected ear facing upwards after a 1 mL of either ITD or ITM had been administered into the middle ear via the ventilating tube. A small volume of medication (1 mL) was used as it offered a better chance of staying in the middle ear and hence ensuring maximal drug exposure. It is thought that larger volumes of medication to be instilled into the middle ear via a grommet would result in undesirable leakage or escaping of fluid and therefore lowering the concentration and exposure of medication in the middle ear. The excess solution was allowed to rest in the external auditory canal. This position had to be maintained for at least thirty minutes after administration of the IT steroid, in order to ensure optimal absorption into the perilymph through the round window membrane. Subsequent IT steroid therapy was performed on a weekly basis in an outpatient clinic setting for a duration of 3 weeks.
Outcome measures
Any improvement was measured by means of PTA, which was performed at (I) initial presentation of ISSNHL, (II) after completion of oral prednisolone treatment in participants receiving oral steroids as an initial therapy, (III) before commencement of IT steroid therapy, (IV) before each subsequent IT steroids administration therapy, (V) on completion of the treatment course, as well as (VI) at 6 and (VII) at 12 months after completion of the treatment.
In each patient, the average of results for three consecutive, most affected frequencies was used for analysis and both subjective and objective measures of hearing were assessed. For the purpose of this study, complete recovery was defined as PTA improvement to within 10 dB of the unaffected ear, no recovery as PTA improvement of less than 10 dB (compared with pretreatment values in the affected ear), and partial recovery as a PTA improvement between these two extremes.
Statistical analysis
The data from each study groups was processed using the GraphPad Prism version 8.0.2 software (Graphpad Software Inc; San Diego, California, USA) and Microsoft Excel version 14.3.2 software (Microsoft Corporation; Redmond, Washington, USA). A paired t-test was used in each study group in order to draw statistical comparisons between PTA results at baseline and post-oral prednisolone therapy or post IT steroid therapy respectively.
Results
Demographic data
High Dose Dexamethasone (ITD) Group
Among the twenty participants, seven received primary ITD therapy and the remainder thirteen patients were given ITD treatment as part of a salvage therapy. Fourteen of the participants were females with and six were males. The patient mean age was 55.3 years.
Among the seven patients who received primary ITD therapy, the mean delay to ITD therapy was 11.9 weeks (Table 1). Salvage patients presented and received treatment slightly earlier with a mean delay to systemic steroids of 4.7 weeks and a mean delay of 10.1 weeks to ITD therapy from onset of ISSNHL (Table 1). The mean delay for all patients to ITD therapy was 10.8 weeks (Table 1). All salvage patients received ITD within 4 weeks of completing oral steroid therapy.
Table 1
| Study parameters | High dose dexamethasone | Methylprednisolone |
|---|---|---|
| Number of participants | 20 | 10 |
| Salvage | 13 | 9 |
| Primary | 7 (35%)§ | 1 (10%)§ |
| Mean age (years) | 55.3 (10.7)*; range [29–68] | 55 (15)*; range [32–75] |
| M:F | 3:7 | 4:1 |
| Mean delay to systemic therapy (weeks) | 4.7 (4.2)*; range 1–16 | 3.6 (4.9)*; range 1 day–12 |
| Mean delay to ITS in primary group (weeks) | 11.9 (4.6)*; range 7–20 | 12 |
| Mean delay to ITS in salvage group (weeks) | 10.1 (4.6)*; range 2–18 | 7.6 (5.5)*; range 2–20 |
| Mean weeks to ITS for combined primary and salvage (weeks) | 10.8 (4.6)*; range 2–20 | 8.0 (5.3)*; range 2–20 |
§, percentage of total number of participants for each group; *, mean (standard deviation). ITS, intratympanic steroids.
High Dose Methylprednisolone (ITM) Group
This group contained more male participants than female counterparts with a ratio of 4:1 (Table 1). The mean age was 55 years. Nine out of the ten participants received ITM as part of a salvage therapy. The mean delay for salvage therapy participant to ITM was 7.6 weeks and the mean for all participants to ITM treatment was 8 weeks (Table 1). Similarly to the ITD group, all participants received ITM therapy within 4 weeks after the completion of high dose oral prednisolone therapy.
Loss to follow-up had been experienced in neither of the groups.
Pre-treatment HL
Various degrees of HL were appreciated in this study and these were classified into six of the following categories based on the frequencies at which HL was the most significant; low, low-mid, mid, mid-high, high, asymmetrical (bimodal) and global HL (Table S1). Table S1 below shows the pattern distribution of HL prior to ITS therapy.
Hearing improvement following IT steroid therapy
High Dose Dexamethasone (ITD) Group
Overall results demonstrated no statistically significant hearing improvement (with P value of 0.79; paired t-test on GraphPad Prism) following high dose ITD therapy when compared to both baseline PTA values and post-oral prednisolone therapy PTA value (Figure 1).
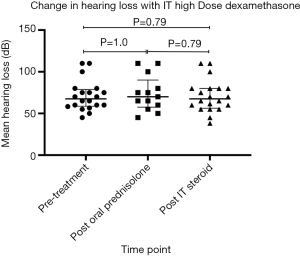
In the salvage group, only one out of the thirteen participants (7.7%) demonstrated partial recovery with an objective improvement of 10 dB after IT dexamethasone therapy (Table 2). Fifteen point four percent demonstrated non statistically significant overall improvement of 5–10 dB after ITD therapy (Table 2). It has also been observed that all of the 3 participants who initially presented with HL in the mid-low frequencies had non-significant minimal or partial hearing recovery. As for the primary group, only one out of seven participants (14.3%) displayed statistically non significant improvement of 3.4 dB (Table S2). Subjective hearing improvement in other participants was not reported.
Table 2
| HL distribution | Total | Improvement (5–10 dB) | Partial recovery (≥10 dB) | Complete recovery | Worsening HL |
|---|---|---|---|---|---|
| Global | 7 | – | – | – | 2 |
| High frequencies | 6 | – | – | – | – |
| Mid-high frequencies | 2 | – | – | – | – |
| Mid frequencies | 1 | – | – | – | 1 |
| Mid-low frequencies | 3 | 2 | 1 | – | – |
| Low frequencies | 1 | – | – | – | – |
| Total | 20 | 2 (15.4%)§ | 1 (7.7%)§ | – | 3 (42.8%)* |
§, percentage of number of salvage participants (N=13); *, percentage of number of primary participants (N=7). HL, hearing loss.
It was furthermore observed that 3 of the primary participants (42.8%) demonstrated worse outcomes with an average worsening of 14.4 dB in the affected ear (Table S2). The affected participants initially presented with moderate-severe HL globally or in the mid-high frequencies. The delay to primary treatment for these 3 participants ranged from 8 to 20 weeks after the onset of HL.
None of the salvage participants experienced worse outcomes.
High Dose Methylprednisolone (ITM) Group
This group revealed a statistically significant improvement in HL following IT methylprednisolone when compared to both pre-treatment and post oral prednisolone therapy PTA values (with P value of 0.0053; paired t-test on Graphpad Prism) (Figure 2).
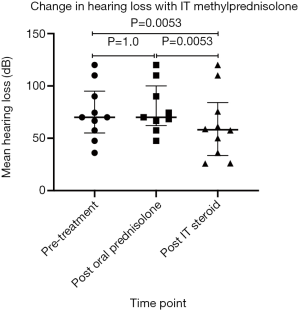
Twenty percent demonstrated a complete recovery of their HL, whereby the HL in the affected ear improved to a difference of less than 10 dB of the unaffected ear (Table 3). These participants were males, who had been started on oral prednisolone therapy and IT methylprednisolone therapy within the second week and the tenth week of onset respectively.
Table 3
| HL distribution | Total | Partial recovery (≥10 dB) | Complete recovery | Worsening HL |
|---|---|---|---|---|
| Global | 5 | 2 | 1 | – |
| Mid-high frequencies | 1 | – | – | – |
| Low frequencies | 1 | 1 | – | – |
| Asymmetric (Bimodal) | 3 | 1 | 1 | – |
| Total | 10 | 4 (40%)* | 2 (20%)* | 0 (0%)* |
*, percentage of total number of participants (N=10).
Overall partial recovery was observed in 40% of the total participants, with improvements in hearing ranging up to 18.5 dB (Table S3). We also noticed that one of the participants with initial mid-high frequency HL, experienced complete recovery in the mid frequencies but no change in the high frequencies.
Modest to no change was observed in the remaining participants who had undergone primary IT steroid treatment or with profound HL, and that despite early initiation of therapy. None of the participants experienced worsening of HL on PTA. Subjective improvement or worsening were not reported.
Associated symptoms
In addition to HL, participants also reported associated symptoms such as vertigo and tinnitus at the time of initial presentation. None reported aural fullness or otalgia initially.
High Dose Dexamethasone (ITD) Group
Among the twenty participants, three experienced vertigo and fourteen had associated tinnitus upon initial presentation. Following completion of ITD treatment, six patients (42.9%) recovered completely from tinnitus and the all of remaining eight patients reported subjective improvement in tinnitus (Table 4). Furthermore, 100% of the participants with initial vertigo, recovered with no residual symptoms post ITD treatment (Table 4). None of the participants experienced side effects (local and systemic) from ITD treatment and no new other symptoms have been reported.
Table 4
| Study parameters | Vertigo | Tinnitus | Pain | Other side effects (local/systemic) |
|---|---|---|---|---|
| IT Dexamethasone Group | ||||
| Pre-ITS treatment | 3 | 14 | 0 | 0 |
| Post-ITS treatment | 0 | 8 | 0 | 0 |
| Recovery of symptoms | 3 (100%)† | 6 (43%)† | 0 | 0 |
| New onset symptoms | 0 | 0 | 0 | 0 |
| IT Methylprednisolone Group | ||||
| Pre-ITS treatment | 2 | 9 | 0 | 0 |
| Post-ITS treatment | 0 | 4 | 3 | 0 |
| Recovery of symptoms | 2 (100%)* | 5 (56%)* | 0 | 0 |
| New onset symptoms | 0 | 0 | 3 (30%)§ | 0 |
†, percentage of participants with initial symptoms; *, percentage of participants with initial symptoms; §, percentage of total number of participants (N=10). ITS, intratympanic steroids.
High Dose Methylprednisolone (ITM) Group
In this group, two and nine of the ten participants experienced vertigo and tinnitus respectively upon initial presentation. Similarly to the dexamethasone group, 100% of the participants with initial vertigo experienced complete recovery. Fifty-six percent of participants recovered from tinnitus, however all reported subjective improvement at the end of the therapy (Table 4). Three participants (30%) developed new onset mild otalgia during IT methylprednisolone therapy (Table 4). However none of the new onset otalgia affected completion of the treatment as they would easily be managed by simple analgesia. Otalgia would typically be present for hours/days following IT methylprednisolone administration. None of the patients reported otalgia at the 12-month mark.
Discussion
Although idiopathic in nature, various aetiologies have been speculated to play a part in the pathogenesis of ISSNHL. Such speculated aetiologies include viral, vascular, endolymphatic hydrops and autoimmune causes; all of which, seem to benefit from the treatment with corticosteroids. It has been theorised that treatment with corticosteroids may have advantages such as decreasing local and systemic inflammation, improving cochlear blood flow as well as regulating inner-ear protein synthesis (14). Despite these benefits, corticosteroids are however often associated with a broad range of side effect profile when given systemically, hence making susceptible individuals less likely to tolerate such treatments. For this reason, the intratympanic route is an appealing alternative as it was found to have a better side effect profile and to be better tolerated, especially in patients for whom the administration of systemic corticosteroids was contraindicated.
This alternative, whether used as a salvage or as a primary treatment, however seems to come with its own set of limitations. It was firstly observed that the efficacy of local corticosteroids was intimately dependent on the time of administration since onset of HL. Studies suggested that better outcomes were achieved if started within the 2 weeks of onset of HL (16). Less benefits is observed if the onset of local intratympanic therapy is delayed to 6 weeks of onset (16). Such delays are sometimes unfortunately inevitable, and these include delays in presentation, referral, workup as well as the delay caused by ventilation tubes insertion to allow intratympanic access. Studies have also suggested that the possible loss of intratympanic medication via the eustachian tube into the nasopharynx, or into the external auditory canal could significantly impede on the improvement rates (3). In addition to this, it was also suggested that patient factors such as middle ear adhesions, could also affect the rates of diffusion of the corticosteroid into the inner ear via the round window (3).
All of these factors could have contributed to a suboptimal outcomes in both groups.
Our study has demonstrated complete recovery in two of the participants with IT methylprednisolone as well as significant improvement in 50% of the remaining participants, whilst also showing partial recovery in only one of the participants in the high dose dexamethasone group. Failure to demonstrate any favourable outcome in HL after the administration of IT high dose dexamethasone, remains incongruent with the current literature on observed hearing improvements after ISSHL treatment with IT high dose dexamethasone. It is thought that this incongruency could be a result of the small sample size, the heterogeneity of patterns, time to treatment as well as the severity of HL at presentation.
While drawing comparisons between the two groups, it was observed that the main determinant factors included (I) a significantly longer delay to systemic oral therapy and IT treatment in the high dose dexamethasone group, with a mean difference of 1.1 and 2.8 weeks respectively; (II) the gender bias between the two groups, whereby the methylprednisolone group contained more male participants than females in contrast to the high dose dexamethasone group; (III) the nature of the HL at presentation, with participants from the dexamethasone group presenting with HL in the higher frequencies. Results from our study demonstrated that participants with mid-low frequencies HL had more favourable outcomes; (IV) the difference in cohort size of 2:1, perhaps contributing to a bias.
In addition, the main limitations of this study are the small sample sizes as well as the lack of a control group. Given our current study parameters, the interpretation of the degree of hearing improvement and its relationship with IT methylprednisolone, hence remains difficult and that, despite the observed marked improvement and recovery in HL with IT methylprednisolone. Therefore, more randomised studies with bigger cohorts across multiple institutions should be carried out in order to further our understanding of the role of intratympanic therapy with dexamethasone and methylprednisolone as a salvage/primary therapy for ISSNHL.
Conclusions
Our study has shown that patients with low-mid frequencies ISSNHL had favourable hearing improvement with either complete hearing recovery or significant partial hearing recovery if ITM was administered up to 8 weeks delay after onset of HL. No significant hearing improvement was observed in the ITD group. Further randomised studies with bigger cohorts and minimal delay to treatment could be carried out in order to evaluate the efficacy of IT steroids in the treatment of ISSNHL.
Table S1
| Pattern of hearing loss | Dexamethasone | Methylprednisolone | |||
|---|---|---|---|---|---|
| Number of participants | Percentage* | Number of participants | Percentage§ | ||
| Global | 7 | 35% | 5 | 50% | |
| High frequencies | 6 | 30% | – | – | |
| Mid-high frequencies | 2 | 10% | 1 | 10% | |
| Mid frequencies | 1 | 5% | – | – | |
| Low-mid frequencies | 3 | 15% | – | – | |
| Low frequencies | 1 | 5% | 1 | 10% | |
| Asymmetrical | – | – | 3 | 30% | |
*, percentage of total number of participants (N=20); §, percentage of total number of participants (N=10).
Table S2
| Participant No. | Pre-treatment | Post oral prednisolone | Post IT Steroids @ 12 months | Difference post IT treatment |
|---|---|---|---|---|
| Global frequencies | ||||
| 1 | 100 | 100 | 100 | 0 |
| 2 | 110 | 110 | 110 | 0 |
| 3 | 65 | 65 | 70 | −5 |
| 4 | 110 | 110 | 110 | 0 |
| 5 | 70 | Primary | 80 | −10 |
| 6 | 60 | Primary | 60 | 0 |
| 7 | 80 | Primary | 93.3 | −13.3 |
| High frequencies | ||||
| 8 | 60 | 60 | 60 | 0 |
| 9 | 56.6 | Primary | 55 | +1.6 |
| 10 | 58.3 | Primary | 56 | +2.3 |
| 11 | 75 | 75 | 75 | 0 |
| 12 | 65 | 65 | 65 | 0 |
| 13 | 55 | 55 | 55 | 0 |
| Mid-high frequencies | ||||
| 14 | 80 | 80 | 80 | 0 |
| 15 | 75 | Primary | 95 | −20 |
| Mid Frequencies | ||||
| 16 | 60 | Primary | 56.6 | +3.4 |
| Mid low frequencies | ||||
| 17 | 50 | 50 | 45 | +5 |
| 18 | 45 | 45 | 38.3 | +6.7 |
| 19 | 75 | 75 | 65 | +10 |
| Low frequencies | ||||
| 20 | 70 | 70 | 70 | 0 |
| Mean (SD) | 71.0 (18.1) | 73.8 (21.3) | 72.0 (20.7) | −0.97 (6.8) |
| Median (IQR) | 67.5 (76.2–59.6) | 70 [80–60] | 67.5 (83.3–56.4) | 0 (1.8–0) |
SD, standard deviation; IQR, interquartile range; PTA, pure tone audiometry.
Table S3
| Participant No. | Pre-treatment | Post oral prednisolone | Post IT steroids @ 12 months | Difference post IT treatment |
|---|---|---|---|---|
| Global frequencies | ||||
| 1 | High: 60 | High: 60 | High: 21.5 | +38.5 |
| Mid: 55 | Mid: 55 | Mid: 30 | +25 | |
| Low: 45 | Low: 45 | Low:25 | +20 | |
| 2 | High: 90 | High: 90 | High: 85 | +5 |
| Mid: 90 | Mid: 90 | Mid: 70 | +20 | |
| Low: 90 | Low:90 | Low: 71.6 | +18.4 | |
| 3 | High: 85 | High: 85 | High: 70 | +15 |
| Mid: 71.6 | Mid: 71.6 | Mid: 63.3 | +8.3 | |
| Low: 66.6 | Low: 66.6 | Low: 50 | +16.6 | |
| 4 | 110 | 110 | 110 | 0 |
| 5 | 120 | 120 | 120 | 0 |
| Mid-high frequencies | ||||
| 6 | High: 80 | High: 80 | High: 80 | 0 |
| Mid: 60 | Mid: 56.7 | Mid: 20 | +40 | |
| Asymmetrical (Bimodal) frequencies | ||||
| 7 | High: 70 | High: 70 | High: 51.5 | +18.5 |
| Low: 70 | Low: 70 | Low: 65 | +5 | |
| 8 | High: 40 | High: 40 | High: 38.8 | +1.2 |
| Low: 55 | Low: 55 | Low: 13.33 | +41.67 | |
| 9 | High: 33.3 | Primary | High: 30 | +3.3 |
| Low: 55 | Primary | Low: 55 | 0 | |
| Low frequencies | ||||
| 10 | 60 | 60 | 55 | +5 |
| *Mean (SD) | 70.3 (21.8) | 73.1 (20.8) | 56.3 (28.4) | 14.1 (13.5) |
| §Median (IQR) | 68.3 (86.3–55) | 70 (88.8–57.5) | 55 (70.4–30) | 11.65 (20–2.8) |
*, standard deviation; §, interquartile range. PTA, pure tone audiometry.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.09.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the participants were thoroughly informed and consented prior to taking part in the study. Of note, this study does not require formal ethics approval as intratympanic steroid as a primary or salvage treatment for ISSNHL has been widely studied and is currently considered to be an effective treatment by many international institutions. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shaia FT, Sheehy JL. Sudden sensori-neural hearing impairment: a report of 1,220 cases. Laryngoscope 1976;86:389-98. [Crossref] [PubMed]
- Foden N, Metha N, Joseph T. Sudden onset hearing loss--causes, investigations and management. Aust Fam Physician 2013;42:641-4. [PubMed]
- Oue S, Jervis-Bardy J, Stepan L, et al. Efficacy of low-dose intratympanic dexamethasone as a salvage treatment for idiopathic sudden sensorineural hearing loss: the Modbury Hospital experience. J Laryngol Otol 2014;128:S27-30. [Crossref] [PubMed]
- Suzuki H, Koizumi H, Ohkubo J, et al. Hearing outcome does not depend on the interval of intratympanic steroid administration in idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol 2016;273:3101-7. [Crossref] [PubMed]
- Eisenman D, Arts HA. Effectiveness of treatment for sudden sensorineural hearing loss. Arch Otolaryngol Head Neck Surg 2000;126:1161-4. [Crossref] [PubMed]
- Li H, Feng G, Wang H, et al. Intratympanic steroid therapy as a salvage treatment for sudden sensorineural hearing loss after failure of conventional therapy: a meta-analysis of randomized, controlled trials. Clin Ther 2015;37:178-87. [Crossref] [PubMed]
- Lee KH, Ryu SH, Lee HM, et al. Is Intratympanic Dexamathasone Injection Effective for the Treatment of Idiopathic Sudden Sensorineural Hearing Loss? J Audiol Otol 2015;19:154-8. [Crossref] [PubMed]
- Erdur O, Kayhan FT, Cirik AA. Effectiveness of intratympanic dexamethasone for refractory sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol 2014;271:1431-6. [Crossref] [PubMed]
- Lavigne P, Lavigne F, Saliba I. Intratympanic corticosteroids injections: a systematic review of literature. Eur Arch Otorhinolaryngol 2016;273:2271-8. [Crossref] [PubMed]
- Hamid M, Trune D. Issues, indications, and controversies regarding intratympanic steroid perfusion. Curr Opin Otolaryngol Head Neck Surg 2008;16:434-40. [Crossref] [PubMed]
- Grewal AS, Nedzelski JM, Chen JM, et al. Dexamethasone uptake in the murine organ of Corti with transtympanic versus systemic administration. J Otolaryngol Head Neck Surg 2013;42:19. [Crossref] [PubMed]
- Light JP, Silverstein H. Transtympanic perfusion: indications and limitations. Curr Opin Otolaryngol Head Neck Surg 2004;12:378-83. [Crossref] [PubMed]
- Ng JH, Ho RC, Cheong CS, et al. Intratympanic steroids as a salvage treatment for sudden sensorineural hearing loss? A meta-analysis. Eur Arch Otorhinolaryngol 2015;272:2777-82. [Crossref] [PubMed]
- Haynes DS, O'Malley M, Cohen S, et al. Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Laryngoscope 2007;117:3-15. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Crossref] [PubMed]
- Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg 2012;146:S1-35. [Crossref] [PubMed]
Cite this article as: Li Sung Sang CCY, Zhen E, Shaw CKL. Evaluating and comparing the efficacy of intratympanic high dose dexamethasone (24 mg/mL) and high dose methylprednisolone (125 mg/mL) as a primary and salvage treatment for idiopathic sudden sensorineural hearing loss. Aust J Otolaryngol 2019;2:26.


