
Modified uvulopalatopharyngoplasty and coblation tongue channelling in the management of obstructive sleep apnoea: a single surgeon’s experience
Introduction
Obstructive sleep apnoea (OSA) is a common chronic condition, with recent studies reporting a prevalence of moderate-to-severe disease (≥ 15 events per hour) in 23% of women and almost 50% of men (1). The common presenting complaints are snoring, daytime sleepiness, fatigue and impaired mood and concentration. OSA is associated with reduced quality of life and adverse cardiovascular and metabolic outcomes, including myocardial infarction, stroke and diabetes (2,3). The pathogenesis of OSA involves collapse of the upper airway during sleep. Severity of OSA is based on apnoea hypopnea index (AHI): an AHI of 5–14 represents mild disease, 15–30 represents moderate disease and greater than 30 represents severe disease. The mainstay of treatment has been positive airway pressure (PAP), with the most common device being continuous positive airway pressure device (CPAP), regarded as the gold standard of care. However, adherence to CPAP can be problematic, with adherence rates varying between 46–83% (4). Adherence rates within the first two weeks of therapy have been shown to predict long term compliance (5). While symptomatic improvements with CPAP are often good, results of the recent SAVE trial indicated that CPAP therapy did not prevent negative cardiovascular outcomes in OSA patients with established cardiovascular disease. Again, adherence may have been a contributing factor to this result - the mean CPAP adherence rate was only 3.3 hours per night. However, this reflected the situation with adherence in clinical practice (6). As an alternative to CPAP, mandibular advancement devices (MAD) have been utilised in the management of mild to moderate OSA. MAD aims to protrude the mandible, displacing the tongue and soft palate anteriorly, thus improving upper airway stabilization. The predominant effect may be enlargement of the velopharyngeal airway calibre, particularly in the lateral dimension (7). However, MAD therapy has been associated with a number of side effects, including temporomandibular joint changes, occlusal changes, incisor inclination and molar positional change, restricting use of such devices to patients with an intact dentition and sufficient alveolar ridge to retain the device. Furthermore, patient selection for MAD remains problematic, with no validated predictors of success.
Recently, surgery in the management of OSA patients has undergone significant improvement in outcomes, with a paradigm shift towards reconstructive techniques, as opposed to ablative techniques. Surgery may present an option to patients who have failed or declined MAD or CPAP, as it provides an alternative way to reduce sleep apnoea severity and treat symptoms. Uvulopalatopharyngoplasty (or its variants) is the most common surgical procedure performed. A recent, landmark Australian multicentre study produced statistically significant improvements in both sleep parameters (AHI, nadir oxygen saturation) and clinical symptoms [Epworth Sleepiness scale (ESS)] when the Australian technique of modified UPPP was combined with Coblation tongue channelling (8). The purpose of this study is to present a single surgeon’s (NWS) experience employing the Australian modified uvulopalatopharyngoplasty and Coblation tongue channelling technique in patients who had either failed or declined CPAP and/or MAD therapy with more severe OSA and further compare the conventional definition of surgical success to changes in AHI, ODI and ESS parameters.
Methods
This a retrospective cohort review of prospectively gathered data. It includes 34 consecutive patients from January 2012–February 2018 who underwent the Australian modified uvulopalatopharyngoplasty and Coblation tongue channelling completed by a single surgeon (NWS). Ethics approval for the project was obtained from the Northern Sydney Local Health District research ethics committee (LNR/16/HAWKE/466) following written consent from the patients. Patients were included if they had failed or declined CPAP or MAD therapy, had confirmed OSA on preoperative polysomnography and were deemed anatomically suitable after thorough physical examination, including endoscopic upper airway examination with dynamic manoeuvres and Friedman staging (9). Patients with Friedman stage 1, a small tongue base and significant tonsil-generated collapse on Muller’s manoeuvre were deemed most suitable. The surgical procedure involved the Robinson type modified uvulopalatopharyngoplasty technique as described previously, in combination with seven port Coblation channelling of the tongue using an Ultra SP wand (Arthrocare, Smith and Nephew) under general anaesthesia (8). In brief, this procedure involves an initial tonsillectomy, followed by superolateral advancement of the posterior pillar and its musculature into a lateral velopharyngeal port, created by removal of the lateral palatal fat pad. Patients remained in hospital overnight for airway monitoring and were discharged the following day on oral antibiotics for 1 week and analgesia for 2 weeks. All patients had a post-operative sleep study at 3 months post operatively, using level 1 polysomnogram. Data included body mass index (BMI), AHI, oxygen desaturation index (ODI), ESS and all studies were reported by a qualified sleep physician.
Patient outcomes were analysed using Wilcoxon matched pair signed rank test, with the primary outcomes measured being AHI, ODI and ESS and the alpha for significance set as P<0.05.
Results
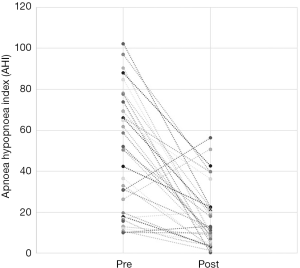
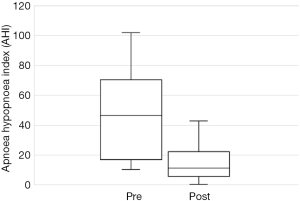
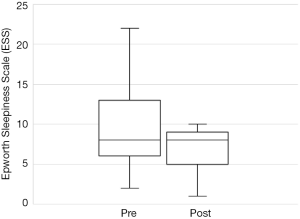
The total number of patients included in the study was 34, with 30 males and 4 females. The mean age of the surgical patients was 36.1 years with a range of 14–67 years. There was no statistically significant difference in BMI between pre and postoperative data (P>0.05). The median preoperative AHI was 46.4 (IQR, 17–70.5) and the median post-operative was 11.1 (IQR, 5.4–22.4). This resulted in a statistically significant difference in median AHI of 29, P<0.05. The median preoperative ODI was 27.6 (IQR, 10–52.5) and the median post-operative ODI was 5.4 (IQR, 2.9–18.7) and this resulted in a median difference in ODI of 15.6 which was also statistically significant (P<0.05). Complete data for Epworth Sleepiness Scales were only available in 19/34 patients. The median preoperative ESS was 8 (IQR, 6–13) and the median post-operative ESS was 8 (IQR, 5–9). Both of these values are in the normal range. Despite the medians being the same, overall the cohort had a statistically significant improvement in ESS (P<0.05).
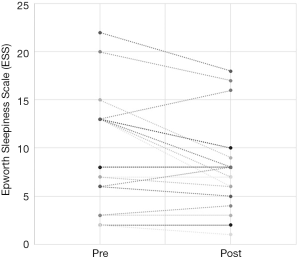
Out of the 34 patients, 19 patients (56%) met the conventional definition of surgical success (that is, a reduction in AHI of greater than 50% and a post-operative AHI less than 20). A subgroup analysis was performed, based on Friedman staging, which has been shown to predict success in uvulopalatopharyngoplasty. Those patients who were classified as Friedman stage 1 had a surgical success rate of 75% (12/16 patients). 3 out of 9 (33%) patients who were Friedman stage 2 had surgical success and 3/6 (50%) of patients who were Friedman stage 3 achieved surgical success. There were three patients who were Friedman stage 4, one of which was surgically successful. A second Friedman stage 4 patient had a reduction in AHI from 88 to 43 (>50%) post operatively and was successfully able to use CPAP following surgery. A summary of the data based on Friedman staging is provided in Table 1. Dot plots have been provided (Figures 1-3) to outline individual differences following surgery, with dashed lines corresponding to the same patient. Similarly, box plots have been used to illustrate the differences in AHI, ODI and ESS found in Figures 4-6 respectively.
Table 1
| Friedman stage | Surgical cure rate | Percent |
|---|---|---|
| 1 | 12/16 | 75 |
| 2 | 3/9 | 33 |
| 3 | 3/6 | 50 |
| 4 | 1/3 | 33 |
| Total | 19/34 | 56 |
*, surgical cure was based on the conventional definition of greater than 50% reduction in AHI and a post-operative AHI <20.


Discussion
OSA is a common chronic medical condition. The presenting symptoms include snoring, daytime sleepiness, fatigue, impaired mood and concentration. Severe OSA is associated with reduced quality of life and increased rates of hypertension, arrhythmias, cardiovascular and cerebrovascular disease, diabetes, cancer and an overall increase in mortality. The first line treatments are generally non-surgical and involve CPAP or MAD. However, many patients remain non-adherent to device treatment and surgery may be considered as a treatment option. Many surgical procedures have been described for the treatment of OSA, but uvulopalatopharyngoplasty is the commonest surgical procedure performed by otorhinolaryngologists. It was first described by Fujita et al. in 1981 (10) and involved excision of the palatine tonsils and removal of redundant soft palate mucosa. Since then, a number of papers have described various modifications of the original procedure, including lateral pharyngoplasty (11), laser assisted uvulopalatoplasty (12) and expansion sphincter pharyngoplasty (13), with varying success rates. More traditional UPPP techniques (without surgically addressing the tongue base) have been the subject of two recent randomised controlled trials. The SKUP3 trial showed mean AHI drop from 52.9 to 23.6 postoperatively at 6 months, which was a statistically significant improvement over the control group (14). A German trial showed mean AHI drop from 33.7 to 15.4 postoperatively at 3 months, which was also a statistically significant improvement over the control group (15). The Australian, Robinson-type modified uvulopalatopharyngoplasty technique, used in our cohort, is a reconstructive procedure which aims to reduce the problems of post-operative scarring and consequent globus sensation and velopharyngeal insufficiency, which beset the original, ablative technique. The Australian technique involves removal of the palatine tonsils and lateral palatal (or “supratonsillar”) fat pad, to create a superolateral velopharyngeal port, into which the posterior tonsillar pillar and its attached muscular buttress can be repositioned, with subsequent enlargement of the retropalatal airway. A partial uvulectomy is sometimes performed. Coblation tongue channelling, a procedure which utilises saline-enhanced radiofrequency ablation through creation of a plasma field, can reduce the size of the base of tongue, which may enlarge or stabilise the retrolingual airway. Previous studies have highlighted the improvement in AHI seen by coblation tongue channelling as a sole procedure (16), and radiofrequency ablation in combination with UPPP (9). Thus, in combination, the modUPPP with Coblation tongue channelling enables multilevel surgery in a single procedure, aiming to enlarge or stabilise both the retropalatal and retrolingual airways. A recent multicentred trial, published by MacKay et al. (8), highlighted the success of the combined procedure and the paradigm shift towards reconstructive multilevel surgery, in carefully selected patients with OSA who had failed device treatment. Our present study applied the same surgical technique to a cohort of patients with more severe OSA (preoperative median AHI of 46), but found a similar reduction in AHI (postoperative median AHI of 11). We believe this provides evidence further that the Australian modUPP and Coblation tongue channelling can be an effective treatment option in patients with severe OSA.
It is important to select patients carefully for this procedure. The preoperative assessment involved assessment of body-mass index, oral and facial examination and endoscopic examination of the upper airway, with dynamic manoeuvres, including the modified Mueller manoeuvre and Woodson hypotonic method. We believe these dynamic manoeuvres are easy and quick to perform and may help to determine the level of obstruction. The modified Mueller manoeuvre involves a forced inspiratory effort against closed nares, with simultaneous nasoendoscopic assessment of the pharyngeal soft tissue to identify sites of collapse. The Woodson hypotonic method similarly allows changes in the airway to be measured independent of physiological variability (17). If predominantly tonsil or palatal collapse occurred with dynamic manoeuvres, we were more likely to offer surgery. However, the greatest guide to surgical selection was the Friedman staging of each patient. The severity of OSA was discussed with each patient in order to manage the patient’s expectations of surgical outcomes, but severe OSA did not preclude undertaking surgery. While we acknowledge it is difficult to interpret data from the small numbers in this study, the most successful outcomes were achieved in patients with a Friedman stage 1, in whom 75% met the conventional definition of surgical success. MacKay et al. (8) produced a success rate of 76% in Friedman stage 1 patients, with the same surgical technique. More traditional UPPP techniques (without tongue surgery) have had similar success to these Australian results. Friedman et al. (9) achieved success in 80% of Friedman stage 1 patients (n=39), with a preoperative mean AHI of 24 dropping to 7 postoperatively. The SKUP 3 study reported 62% success at 6 months postoperatively, in Friedman stage 1 patients (18). Our Friedman stage 2 patients achieved a success rate of 33%. One patient in this group had previously had laser UPPP, resulting in a significant amount of palatal scarring, which proved difficult to correct surgically. One other patient in this group achieved a reduction in AHI of greater than 50% (preoperative AHI was 78) however their post-operative AHI remained just above 20 (post-operative AHI was 22) which rendered them “unsuccessful” by the conventional definition, even though their clinical symptoms had improved. Success was achieved in half of our Friedman stage 3 patients and one third of the Friedman stage 4 patients in our study. Interestingly, both of the “unsuccessful” Friedman stage 4 patients still achieved a significant reduction in AHI (preoperative AHIs were 102 and 88 and fell to 23 and 43 respectively) and one was subsequently able to use CPAP. While this represents a surgical failure, we would argue this is treatment success.
While AHI is the most important measure of OSA severity and is central to the conventional definition of surgical success, we also examined changes in ODI. This is thought to correlate to cardiovascular risk in OSA. In a recent analysis of 6,106 adults participating in the Sleep Heart Health study, ODI (defined as hypopneas accompanied by oxyhemoglobin desaturation of greater than or equal to 4%) were associated with prevalent cardiovascular disease and this was found to be independent of confounding covariates (19). Similarly, in their study of 267 individuals, Seif et al. (20), found that an ODI above 24.6 was significantly associated with endothelial dysfunction and hence cardiovascular risk, highlighting the importance of this parameter in assessing physiological disease risk in patients with OSA. In our study, the median preoperative ODI was 27.6 (IQR, 10–52.5) and the median post-operative ODI was 5.4 (IQR, 2.9–18.7), a statistically significant improvement. Obviously, in this small case series, no conclusions about the impact of surgery upon cardiovascular risk are able to be drawn. Likewise, the SKUP3 trial showed mean ODI had dropped from 43 preoperatively to 16, at 6 months postoperatively (14). In future, larger studies, ODI may prove to be a useful marker of cardiovascular outcomes in OSA.
The ESS is a simple, self-administered eight-item questionnaire used to assess daytime sleepiness in OSA. A score greater than ten is considered abnormal (21). Previous studies have found a weak correlation between ESS and clinical severity, as measured as AHI, primarily in those with severe OSA (22). Unfortunately, no conclusions can be drawn from our data on the ESS. Complete data was only available for just over half the patients and the preoperative median value of 8 was in the normal range. Figure 3 illustrates that only 3 patients had a worse postoperative score, the other 16 having similar or better postoperative scores. Previous studies have shown improvements in ESS after UPPP or its variants. Browaldh et al. (23) in their prospective randomized controlled trial of 65 patients undergoing UPPP found that it was effective in improving both daytime sleepiness and quality of life. The median ESS was 13 preoperatively and 6.5 postoperatively at 6 months. Likewise, the German randomized controlled trial (15) showed an improvement in ESS from 10.6 preoperatively to 6.2 at 3 months postoperatively, a statistically significant improvement over the control group. Patel et al. (24) found the minimal clinically important difference in ESS to be between −2 and −3, so the results from the SKUP3 and German trials were convincing. Similar results were reported with the Australian modUPPP and Coblation tongue channelling technique in Mackay et al. (8), with median ESS values dropping from 10.5 preoperatively to 5 postoperatively.
Some surgical patients in our study achieved substantial reductions in AHI, representing worthwhile reductions in disease burden, but did not meet the conventional criteria for surgical success. For example, one of our patients achieved a reduction in AHI from 102 to 23, with an associated marked improvement in their ODI from 72 to 19, but was counted as a surgical failure under the conventional definition. One other surgical failure was successful in adhering to CPAP and another was successful in adhering to MAD therapy, which had not been possible prior to surgery. Thus, while surgery may leave residual OSA, this residual disease may be successfully treated with device therapy in some patients. We believe this demonstrates that in some cases, surgical “failure” may still lead to an overall treatment success, by enabling device treatment. Longer term follow is required to ensure the improvements demonstrated across all parameters are maintained.
The future of sleep surgery holds much promise, with evolving techniques, such as hypoglossal nerve stimulation, being assessed as treatment modalities. Despite the retrospective nature of our study limited by a small number of patients, these results add to the evidence that the Australian modified UPPP and Coblation tongue channelling should be considered in OSA patients, even with severe disease, who have failed CPAP or MAD treatment and may provide improvements not only in AHI, but symptoms and cardiovascular disease markers. We argue these factors should be incorporated into the definition of surgical success in the future.
Conclusions
OSA is a condition associated with a significant burden of disease, impaired quality of life and increased cardiovascular and health risks. The results of this study illustrate, that multilevel surgery in the form of the modified uvulopalatopharyngoplasty and Coblation tongue channelling procedure can improve disease severity, symptoms and markers of cardiovascular disease. Therefore, we recommend, that it should be considered in carefully selected patients who have failed CPAP or mandibular device treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.12.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval for the project was obtained from the Northern Sydney Local Health District research ethics committee (LNR/16/HAWKE/466) following written consent from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310-8. [Crossref] [PubMed]
- Marshall NS, Wong KK, Phillips CL, et al. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med 2009;5:15-20. [PubMed]
- Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis 2009;51:434-51. [Crossref] [PubMed]
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective therapy. Proc Am Thorac Soc 2008;5:173-8. [Crossref] [PubMed]
- Popescu G, Latham M, Allgar V, et al. Continuous positive airway pressure for sleep apnoea/hypopnoea syndrome: usefulness of a 2 week trial to identify factors associated with long term use. Thorax 2001;56:727-33. [Crossref] [PubMed]
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnoea. N Engl J Med 2016;375:919-31. [Crossref] [PubMed]
- Chan AS, Sutherland K, Schwab RJ, et al. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax 2010;65:726-32. [Crossref] [PubMed]
- MacKay SG, Carney SA, Woods C, et al. Modified Uvulopalatopharyngoplasty and coblation channelling of the tongue for obstructive sleep apnoea: a multicentre Australian trial. J Clin Sleep Med 2013;9:117-24. [PubMed]
- Friedman M, Ibrahim H, Joseph NJ. Staging of obstructive sleep Apnea/Hypopnea Sydnrome: A guide to appropriate treatment. Laryngoscope 2004;114:454-9. [Crossref] [PubMed]
- Fujita S, Conway W, Zorick F, et al. Surgical correction of anatomic azbnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981;89:923-34. [Crossref] [PubMed]
- Cahali MB, Formigoni GG, Gebrim EM, et al. Lateral pharyngoplasty versus Uvulopalatopharyngoplasty: a clinical, polysomnographic and computed tomography measurement comparison. Sleep 2004;27:942-50. [Crossref] [PubMed]
- Berger G, Stein G, Ophir D, et al. Is there a better way to do laser assisted uvulopalatoplasty? Arch Otolaryngol Head Neck Surg 2003;129:447-53. [Crossref] [PubMed]
- Pang KP, Woodson BT. Expansion sphincter pharnygoplasty: a new technique for the treatment of obstructive sleep apnoea. Otolaryngol Head Neck Surg 2007;137:110-4. [Crossref] [PubMed]
- Browaldh N, Nerfeldft P, Lysdahl M, et al. SKUP 3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea. Thorax 2013;68:846-53. [Crossref] [PubMed]
- Sommer UJ, Heiser C, Gahleitner C, et al. Tonsillectomy with uvulopalatopharyngoplasty in obstructive sleep apnea - a two center randomized controlled trial. Dtsch Arztebl Int 2016;113:1-8.
- Zhang Q, Wang H. Radiofrequency coblation of tongue channeling for supine position associated obstructive sleep apnea. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013;27:768-70.
- Woodson BT, Feroah T, Connolly LA, et al. A method to evaluate upper airway mechanics following intervention in snorers. Am J Otolaryngol 1997;18:306-14. [Crossref] [PubMed]
- Browaldh N, Bring J, Friberg D. SKUP 3: 6 and 24 months follow-up of changes in respiration and sleepiness after modified UPPP. The Laryngoscope 2018;128:1238-44. [Crossref] [PubMed]
- Punjabi NM, Newman AB, Young TB, et al. Sleep disordered breathing and cardiovascular disease: an outcome based definition of hypopneas. Am J Respir Crit Care Med 2008;177:1150-5. [Crossref] [PubMed]
- Seif F, Patel SR, Walia H, et al. Association between obstructive sleep apnoea severity and endothelial dysfunction in an increased background of cardiovascular burden. J Sleep Res 2013;22:443-51. [Crossref] [PubMed]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540-5. [Crossref] [PubMed]
- Kaminska M, Jobin V, Mayer P, et al. The Epworth Sleepiness Scale: Self-administration versus administration by the physician, and validation of a French version. Can Respir J 2010;17:e27-34. [Crossref] [PubMed]
- Browaldh N, Bring J, Friberg D. SKUP (3) RCT; continuous study: changes in sleepiness and quality of life after modified UPPP. Laryngoscope 2016;126:1484-91. [Crossref] [PubMed]
- Patel S, Kon SSC, Nolan CM, et al. The Epworth sleepiness scale: Minimum clinically important difference in obstructive sleep apnea. Am J Respir Crit Care Med 2018;197:961-3. [Crossref] [PubMed]
Cite this article as: Sarkis LM, Lee D, Grunstein R, Stow NW. Modified uvulopalatopharyngoplasty and coblation tongue channelling in the management of obstructive sleep apnoea: a single surgeon’s experience. Aust J Otolaryngol 2019;2:30.


