
Acute food bolus ingestion: ten-year experience at a tertiary pediatric hospital
Introduction
Paediatric foreign body ingestion and impaction is a common problem. Of at least 100,000 cases of foreign body ingestion reported annually in the United States, 80% occur in children (1)—usually between the ages of six months to three years. While most impacted foreign bodies are coins (2,3), button batteries and small toys also commonly impact in the upper aerodigestive tract, particularly in the upper oesophagus (4). The estimated incidence of oesophageal food bolus impaction in adults is estimated to be approximately 13 cases per 100,000.
By comparison, acute food bolus impaction (AFBI) is an uncommon problem. AFBI is defined as abrupt dysphagia following food ingestion that prevents further passage of food and requires urgent removal (5). A number of anatomical and physiological conditions may predispose a child to developing AFBI. These may include strictures, Shatzki rings, achalasia, extrinsic esophageal compression, dysmotility or reflux disease.
As a result, the causes of AFBI is believed to differ significantly in children compared to that of adults. However, there have been few studies examining the causes and frequency of AFBI in this cohort of patients, particularly in a tertiary paediatric centre where the patient demographic may include complex patients with significant comorbidities.
This study therefore aimed to examine the current presentation and management of AFBI in children at an Australian tertiary paediatric hospital. We also aimed to determine the underlying aetiology or the investigations undertaken to identify the aetiology causing AFBI.
Methods
Study design
All consecutive cases of foreign body ingestion at a tertiary paediatric hospital in Sydney, Australia (Children’s Hospital at Westmead) between January 1994 and December 2004 were retrospectively identified using hospital database searches for International Coding of Diseases 9 (ICD-9) codes 933, 935.1, 935.2, 936, 938 and ICD Ten codes T17.2, T18.1 T18.2, T18.3, T18.4, and T18.9. Cases were individually reviewed and all cases with suspected foreign bodies other than AFBI were excluded.
Data collection
Ethical approval was obtained prior to data collection from the Western Sydney Local Health District Human Research Ethics Committee (WSLHD HREC, approval number MR-2004-12-02).
Physical and electronic records of each case from admission, operation notes and follow-up appointments were reviewed. The following data was collected: demographic data including patient age and sex; whether the child was symptomatic at time of review and the nature of the presenting symptoms; the use of investigations such as X-ray or barium swallow; use of medical management such as glucagon; operative findings, the nature of the food bolus, location of impaction and complications; and the presence of any pre-disposing conditions.
Data analysis
Descriptive data analysis was performed. Categorical variables were reported using percentages and raw numbers. Parametric continuous variables were reported using means with standard deviations. Pearson’s chi-square was performed for comparison between categorical variables.
Data analysis was performed using Statistical Analysis Software 9.4 (SAS Institute, Cary, NC, USA).
Results
A total of 46 children with mean age 6.0±3.4 years, 28.3% (n=13) female, presenting with possible oesophageal AFBI were identified over the 10-year period. 80.4% (n=37) of children presented with a suspected meat bolus while the remaining 19.6% (n=9) presented with suspected vegetable bolus.
All 46 children experienced at least one symptom at presentation. The most common symptoms were vomiting (54.3%, n=25), drooling (34.8%, n=16), dysphagia (32.6%, n=15), refusal to eat (23.9%, n=11) and foreign body sensation (17.4%, n=8).
Twenty-six children underwent plain film X-ray investigation of which 6 were found to be positive for impacted food bolus. 6 children underwent barium swallow, all of which were positive for impacted food bolus (Figure 1).
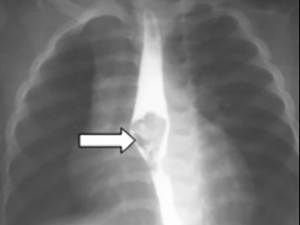
About 73.9% of children (n=34) who presented were found to have an impacted food bolus at time of examination or intervention. The remaining 12 patients were found to have no food bolus or had passed at time of oesophagoscopy. The most common site of impaction was at the lower oesophagus (55.9%, n=19), followed by the upper oesophagus or oropharynx.
Few children (8.8%, n=3) underwent initial medical management with glucagon. Of these, only one passed the food bolus without further intervention.
The remaining 33 children all required endoscopy for removal of the food bolus or to assist distal passage. Most children underwent rigid oesophagoscopy (93.9%, n=31), while the remaining few underwent direct laryngoscopy (6.1%, n=2). Both methods were successful in all attempts to retrieve or dislodge the food bolus, if one was identified. Those who underwent rigid oesophagoscopy were noted to have significant mucosal erosion or bleeding following retrieval, although these were managed expectantly with no further complications.
More than half (52.2%, n=24) of the presenting cohort were noted to have previous oesophageal surgery or significant pre-existing condition involving the oesophagus. The majority (75.0%, n=18) of these underwent previous closure of trachea-oesophageal fistula, while a few patients (8.3%, n=2) also required correction of oesophageal atresia. The remaining (8.3%, n=2) patients had a previous confirmed history of eosinophilic oesophagitis (EoE).
Of the remaining 22 (47.8%) patients without known pre-disposing conditions, no records of further investigations or mucosal biopsies were identified.
Discussion
Emergency presentation for AFBI is an uncommon problem in adults and even less common in children. In adults, AFBI is mostly seen in the elderly, typically resulting from an acquired oesophageal dysmotility or reflux-related oesophageal disease.
All patients in our cohort were symptomatic at time of presentation and most patients had a demonstrable food bolus impaction in situ at time of review. X-ray imaging, as expected, was a poor diagnostic tool for the detection of food bolus. Barium swallow, while demonstrating greater diagnostic accuracy, was used rarely in our cohort. The authors suggest that forcing children to drink contrast when a food bolus causing obstruction is present causes patient and carer distress, and should be reserved only when there is suspicion of perforation or to assess stenosis.
As a result, the authors recommend endoscopic examination in the first instance for any symptomatic child with suspected AFBI. Not only is endoscopy highly sensitive and specific for the diagnosis of an impacted food bolus, it allows inspection and biopsy of the lumen of the oesophagus for underlying pathology and immediate management of the impacted bolus by retrieval or dislodgement to facilitate spontaneous passage.
Glucagon trial was infrequently used in our series. Glucagon has been previously used to induce relaxation of the distal oesophagus. However, recent studies have demonstrated a success rate of glucagon of under 50%, which decreases in patients with structural abnormalities (6). A small randomized controlled trial by Tibbling et al. (7) did not demonstrate difference in disimpaction rate when compared to placebo. Furthermore, intravenous glucagon may also be associated with adverse effects such as nausea and vomiting, potentially risking further injury. Nevertheless, it appears further research is required to determine the real-world effectiveness of glucagon in this setting.
In our cohort of children presenting with AFBI, approximately half had a history of previous oesophageal surgery and subsequent dysmotility—a widely recognised predisposing factor to oesophageal stricture formation. The preferred management plan in management AFBI in children with previous TOF with potential tracheal stenosis is centred around several considerations. In the first instance, a complete history should be undertaken to ascertain if the patient may have oesophageal stenosis or dysfunction, with questioning directed toward previous food or drink consumption, and any previous history of dysphagia or AFBI episodes. Oesophagoscopy should then be performed to remove the foreign body where possible, with specific care not to induce oesophageal rupture. The method of oesophagoscopy should depend on the technical expertise of the surgeon and their level of comfort with the procedure, although a flexible oesophagoscope may confer advantages in cases where there is limited view of the oesophagus. Following removal, re-examination of the site of previous oesophageal repair should be performed to identify evidence of oesophageal stenosis or stricture. In some cases, radiological or contrast-enhanced assessment can be performed to assess for oesophageal stenosis. Oesophageal stenosis, where present, should be manages accordingly. Assessment of pH proven reflux and subsequent management if present with proton pump inhibitors and other anti-reflux measures should be pursued. At the authors’ institution, a bougie is never used when a foreign body is still in place, as it is believed that this increases the risk of rupture.
The remaining children with a known predisposing factor were noted to have previous history of EoE. This finding is consistent with recent evidence demonstrating that EoE is a likely culprit for many presentations of paediatric AFBI (8). In the other cases of AFBI where no cause was found, further investigation or mucosal biopsy was not pursued, likely due to the fact awareness that EoE as a potential contributing factor had not been widely established at the time the cohort presented.
Recently, there has been increasing recognition that EoE, a chronic allergic inflammatory condition involving the oesophagus, is a risk factor for AFBI in both adults and children (9,10). While current understanding remains limited, EoE is believed to be a chronic disease involving both genetic and environmental factors. It is believed that protein antigens from various foods cause an allergic response that, over time, leads to chronic inflammation, eosinophilic infiltration and deposition of subepithelial fibrous tissue. Oesophageal remodelling and dysfunction may result in luminal strictures, leading to symptoms that mimic gastro-oesophageal reflux disease and predispose to AFBI (11).
Diagnosis of EoE is typically made on the recognition of symptoms, and a high eosinophil count on oesophageal biopsy (12). Treatment involves dietary avoidance of known triggers, supplemented by allergy testing, and topical corticosteroids (11). Where conservative measures fail, such as in non-compliance of medications; or where delivery is challenging (such as with poor inhaler technique) or when stenosis is critical, surgical dilatation may provide short term symptomatic benefit (13).
EoE is defined is characterised by specific symptoms, oesophageal dysfunction and predominantly eosinophilic inflammatory infiltrate (Figure 2). Symptoms in children include dysphagia, abdominal pain and reflux-mimicking symptoms such as heartburn (14). Prevalence of EoE has continued to increase in the last decade, secondary to increasing awareness of the condition (9). EoE has been described as a disease affecting younger populations, with a general predisposition towards males, who account for up to 81% of cases of EoE. As approximately 70% of our cohort was male, we speculate that at least some of these children may have had undiagnosed EoE.
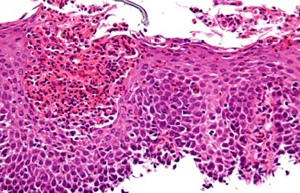
In a 2012 study, El-Matary et al. (8) found that, in a cohort of 140 children with foreign body impaction, 39% (n=7 out of 18) of those with AFBI had EoE, compared to 3% in children with other (non-food bolus) foreign body impactions. Given these findings, they suggested that routine sampling of oesophageal mucosa during oesophageal food bolus FB extraction may be considered to assess for the presence of EoE.
A systematic review and meta-analysis performed by Hiremath et al. (15) of 14 studies demonstrated that oesophageal biopsies were obtained from only 54% of individuals presenting with AFBI, and of these only 54% were positive for EoE. However, studies carried significant heterogeneity, with varying cohort ages, locations, subspecialty attending teams and adherence to the current EoE diagnostic criteria for determination of a positive biopsy. Overall, they concluded that while it is highly plausible that EoE is a major risk factor for AFBI, the quality of current evidence linking the two is limited given the above considerations.
However, given the results of the current study and on review of the literature, it is the authors’ opinion that in all children presenting with AFBI, investigation of EoE with oesophageal biopsy should be pursued unless there are specific contraindications. Our current practice is to perform the biopsies with gastroscopy biopsy forceps at each of the lower, middle and upper oesophagus. Gastroscopy biopsy forceps are used in favour of standard oesophageal forceps, which are more traumatic and may leave linear lacerations that can progress into major tears and mediastinitis. This may be performed either during the acute episode, or on follow-up, alongside referral to a gastroenterologist, if appropriate. A diagnosis of EoE should be based on the accepted consensus recommendations (16).
The decision to use rigid or flexible endoscopy for retrieving foreign bodies in children or adults are dependent on the technical experience of the endoscopists. Russell et al. (17) demonstrated that most foreign bodies are found in the upper oesophagus (78%) and removal can be undertaken either with the rigid or flexible oesophagoscope with the correct accessory device for removing the foreign body. They found that 97% of foreign bodies can be removed with either device, but in 3%, the ability to use the alternative method covers those found to be more difficult to remove. Therefore, an appropriate algorithm of managing oesophageal foreign bodies for any unit should incorporate the skills of the treating specialists at that unit. In some units, the endoscopy service utilises a multidisciplinary model where one clinician is expert at one technique and the other at the other technique. This may enable management of a foreign body at the lower third of the oesophagus where the rigid oesophagoscope may be difficult to use, such as in a patient with abnormal anatomy, with cervicothoracic scoliosis/kyphosis; or the older child where the oesophagoscope is too short. It is the authors’ opinion then, that with treatment failures, the ability to remove the foreign body with either device in the unit allows a more complete service in the case of potential treatment failure and to avoid potential complications of any form of endoscopy which may lead to a prolonged hospital stay or other morbidity.
While there are some suggestive features of EoE on endoscopy such as ridges or furrows (trachealisation or felinization) or on barium swallow such as rings (Figure 3) mucosal irregularity, stricture formation or a narrow caliber esophagus (18), patients with EoE may have a normal appearing oesophagus. A systematic review and meta-analysis performed by Kim et al. (13) demonstrated only a modest overall sensitivity of endoscopic examination for EoE ranging from 15% to 48%, while overall specificity was greater from 90–95%. As a result, they concluded that endoscopic findings alone are inadequate for the diagnosis of EoE and supported that biopsy specimens should be obtained regardless of endoscopic findings.
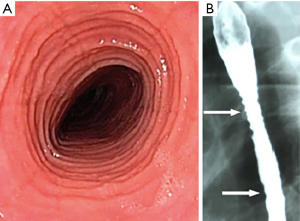
From the results of this study and the review of the literature, the authors suggest that patients who present with AFBI should follow an established clinical pathway at tertiary institutions, with the involvement of both otolaryngology and gastroenterology teams. Specific history-taking regarding previous TOF repair should be undertaken with all children, while children without other obvious causes should undergo routine testing for EoE. While oesophageal biopsy remains a critical aspect of EoE diagnosis, other adjunctive investigations can be considered, including serum IgE, radiography, oesophageal physiology studies and exclusion of GORD. Where diagnosis of EoE is positive, long term therapy and management should be guided by a pediatric gastroenterologist.
Nevertheless, further study is warranted to improve the provision of care for patients presenting with AFBI. High quality studies are required to determine if adjunctive treatments such as glucagon or hyoscine is recommended in the emergency setting, and whether a “watch-and wait” approach rather than direct surgical intervention may be appropriate in a subset of patients.
Conclusions
AFBI is an uncommon presentation in paediatric populations and is usually associated with an underlying predisposing condition. Clinicians should maintain a low threshold for endoscopy, which is not only useful for diagnosis and treatment, but to facilitate investigations for an underlying cause. Given that eosinophilic oesophagitis has recently been identified as a potential risk factor both in this study and in the literature, investigation for EoE with oesophageal biopsies should be considered for any child who presents with AFBI. Future studies are required to optimize treatment paradigms for this population of patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.12.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained prior to data collection from the Western Sydney Local Health District Human Research Ethics Committee (WSLHD HREC, approval number MR-2004-12-02). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wyllie R. Foreign bodies in the gastrointestinal tract. Curr Opin Pediatr 2006;18:563-4. [Crossref] [PubMed]
- Little DC, Shah SR, St Peter SD, et al. Esophageal foreign bodies in the pediatric population: our first 500 cases. J Pediatr Surg 2006;41:914-8. [Crossref] [PubMed]
- Waltzman ML, Baskin M, Wypij D, et al. A randomized clinical trial of the management of esophageal coins in children. Pediatrics 2005;116:614-9. [Crossref] [PubMed]
- Singh N, Chong J, Ho J, et al. Predictive factors associated with spontaneous passage of coins: A ten-year analysis of paediatric coin ingestion in Australia. Int J Pediatr Otorhinolaryngol 2018;113:266-71. [Crossref] [PubMed]
- Lao J, Bostwick HE, Berezin S, Halata MS, Newman LJ, Medow MS. Esophageal food impaction in children. Pediatr Emerg Care 2003;19:402-7. [Crossref] [PubMed]
- Ko HH, Enns R. Review of food bolus management. Can J Gastroenterol 2008;22:805-8. [Crossref] [PubMed]
- Tibbling L, Bjorkhoel A, Jansson E, et al. Effect of spasmolytic drugs on esophageal foreign bodies. Dysphagia 1995;10:126-7. [Crossref] [PubMed]
- El-Matary W, El-Hakim H, Popel J. Eosinophilic esophagitis in children needing emergency endoscopy for foreign body and food bolus impaction. Pediatr Emerg Care 2012;28:611-3. [Crossref] [PubMed]
- Mahesh VN, Holloway RH, Nguyen NQ. Changing epidemiology of food bolus impaction: is eosinophilic esophagitis to blame?. J Gastroenterol Hepatol 2013;28:963-6. [Crossref] [PubMed]
- Diniz LO, Towbin AJ. Causes of esophageal food bolus impaction in the pediatric population. Dig Dis Sci 2012;57:690-3. [Crossref] [PubMed]
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007;133:1342-63. [Crossref] [PubMed]
- Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108:679-92; quiz 693. [Crossref] [PubMed]
- Kim HP, Vance RB, Shaheen NJ, et al. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:988-96.e5. [Crossref] [PubMed]
- Liacouras CA. Clinical presentation and treatment of pediatric patients with eosinophilic esophagitis. Gastroenterol Hepatol (N Y) 2011;7:264-7. [PubMed]
- Hiremath GS, Hameed F, Pacheco A, et al. Esophageal Food Impaction and Eosinophilic Esophagitis: A Retrospective Study, Systematic Review, and Meta-Analysis. Dig Dis Sci 2015;60:3181-93. [Crossref] [PubMed]
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:3-20.e6; quiz 21-2.
- Russell R, Lucas A, Johnson J, et al. Extraction of esophageal foreign bodies in children: rigid versus flexible endoscopy. Pediatr Surg Int 2014;30:417-22. [Crossref] [PubMed]
- Al-Hussaini A. AboZeid A, Hai A. How does esophagus look on barium esophagram in pediatric eosinophilic esophagitis?. Abdom Radiol (NY) 2016;41:1466-73. [Crossref] [PubMed]
Cite this article as: Wong E, Cheng AT, Aggarwala S, Cope D, Chong J, Duvnjak M, Singh N. Acute food bolus ingestion: ten-year experience at a tertiary pediatric hospital. Aust J Otolaryngol 2020;3:1.


