
Efficacy of anti-biofilm gel, chitogel-mupirocin-budesonide in a sheep sinusitis model
Introduction
Recalcitrant chronic rhinosinusitis (CRS) has been increasingly recognised to be attributed to bacterial biofilms (1-5). Amongst surgically recalcitrant patients about 40–50% of biofilms identified are Staphylococcus aureus (6,7). Oral antibiotics are often ineffective against biofilms (8), pressuring a continuous search for topical anti-biofilm agents which allows for increased concentration, localised action and less systemic side effects.
The effective delivery of these topical treatments into sinus cavities remains the main obstacle. Currently topical antibiotics are administered via sinonasal irrigations, nebulisers and nasal sprays. All of these methods lack the ideal characteristics of complete sinus distribution, prolonged mucosal contact time to increase local absorption and minimal waste (9). Patients receive highly variable drug penetration influenced by patient condition, sinus surgery, delivery devices, irrigation volume and pressure, and patient positioning (9-12). To overcome all these challenges, we looked at investigating the efficacy of CG (chitosan and dextran), a surgical hydrogel FDA approved for the use after sinus surgery, acting as a drug carrier to deliver the anti-inflammatory effects of budesonide and antibiofilm effects of mupirocin in a previously validated S. aureus biofilm sheep sinusitis model (13,14).
The aim of this study was to (I) optimize a sheep sinusitis model for the investigation of a topical gel treatment and (II) to evaluate the antibiofilm and anti-inflammatory effects of CG-budesonide-mupirocin (CG-BM) in the treatment of S. aureus biofilms in vivo.
Methods
Ethics approval was obtained from the Animal Ethics Committee of The University of Adelaide and the South Australian Health and Medical Research Institute (SAHMRI). All reports on animal experiments have been conducted in accordance with institutional and national guidelines for the care and use of laboratory animals.
Optimisation arm
A total of 5 male merino sheep heads were obtained from the Murray Bridge Abattoir, South Australia. To achieve optimum retention of the gel within the frontal sinuses for 5–7 days, we optimised the method of gel application and determined the appropriate volume of saline flush to be 15 mL twice a day commencing 24 hours after gel instillation.
Study arm
Animals
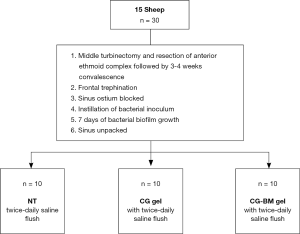
A total of 15 male merino sheep between the dental age of 2 to 4 years were used. All animals were drenched to eradicate the parasite Oestrus Ovis.
Bacterial inoculum
Reference strain American Type Culture Collection (ATCC) 25923 Staphylococcus aureus, known to be biofilm forming, was supplied by the Queen Elizabeth Hospital Department of Microbiology, Adelaide. Frozen glycerol stock was defrosted and subcultured for 24 hours in 3mL of nutrient broth (Oxoid, Adelaide, Australia) on a shaker at 37 °C before inoculum was transferred to a 1% nutrient agar plate (Oxoid, Adelaide, Australia). The plate was incubated for 16–18 hours at 37 °C and a single colony forming unit (CFU) was diluted to 0.5 McFarland standard in 0.45% sterile saline and transferred on ice for instillation into sheep sinuses.
CG
CG comprised of three components; 5% succinyl-chitosan, 0.3% phosphate buffer and 3% dextran aldehyde (Chitogel®, Wellington). All components were manufactured and sterilised by Chitogel® and cultured for sterility by the Department of Microbiology, Princess Margaret Hospital, Western Australia prior to being used in this study. All stocks were stored at room temperature.
Preparation of CG
Dextran aldehyde was first dissolved in 10 mL of phosphate buffer then mixed with 10 mL of succinyl-chitosan using sterile technique.
Preparation of CG-BM
Ten mg of powdered mupirocin (PCCA, Houston) was solubilised under sterile conditions in 6 mL of phosphate buffer 24 hours before application. Mupirocin solution was mixed with 2 mg/4 mL of Pulmicort respules (AstraZeneca Ab, Sodertalje) before being used to dissolve dextran aldehyde and then mixed with 10mL of succinyl-chitosan using sterile technique.
Anaesthetic protocol
All sheep were fasted for 12 hours prior to general anaesthesia. They were induced with intravenous phenobarbitone (19 mg/kg), intubated and placed onto 1.5% to 2% inhalation isoflurane over the course of the procedure. All sheep were placed supine on a wooden cradle and supported with neck slightly flexed on a head ring. Two sprays of Cophenylcaine Forte (ENT Technologies Pty Ltd, Australia) were applied to each nasal cavity 10 minutes prior to procedure.
Surgical protocol
As per protocol (13,14) all sheep had middle turbinectomy and anterior ethmoid complex resection followed by a 3–4 week recovery. Then frontal mini-trephines were placed bilaterally on the sheep’s forehead, 1cm lateral from the midline at the level of superior orbital rims. Accurate trephine placements were verified when fluorescein flushed via trephines (0.1 mL diluted in 100 mL of normal saline) can be visualised endoscopically draining from the frontal sinus ostium into nasal cavity.
Efficacy arm
After frontal trephination, petroleum gauze (Vaseline, Kendall, Mansfield, MA, USA) were used to pack the frontal ostiums. One mL of 0.5 McFarland Units S. aureus was instilled via mini-trephines into each sinus cavity and biofilms were allowed to form over 7 days. On day 8, the petroleum gauze was unpacked and each sheep was randomly assigned into one of three efficacy groups: (I) twice-daily NT, (II) CG and (III) CG-BM. For sheep assigned to CG and CG-BM groups, the gels were instilled once via mini-trephines into each sinus cavity until extrusion visualised under direct endoscopic view from the frontal sinus ostium. All sheep received sinus irrigations with 15 mL of sterile normal saline twice a day commencing 24 hours after gel application for a total of 6 days. Sinus irrigations were commenced 24 hours later based on protocol of previous clinical studies (15,16). All sheep were euthanised on day 8 and sinus mucosa harvested for histopathological analysis and biofilm biomass imaging (Figure 1).
Biofilm imaging
From each sinus cavity, two random sections of 1 cm × 1 cm sinus mucosa were sampled and briefly immersed in phosphate buffered solution to wash off planktonic cells. Sampled mucosa was then stained with LIVE/DEAD BacLight stain (Life Technology, Mulgrave, Victoria, Australia) as per manufacturer’s instructions. Confocal scanning laser microscope (Zeiss Germany) were used to assess biofilm biomass. Three Z-stack images of highest biofilm presence were taken of each sample (Image properties: line average 4, 512×512 pixels, Z-stack 80 steps) making a total of 6 Z-stack images per sinus. COMSTAT2 software (Lyngby, Denmark) was later utilised to quantify biofilm biomass from each Z-stack.
Histopathology evaluation
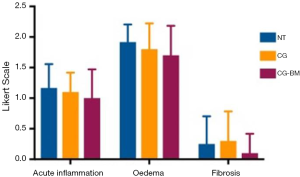
From each sinus cavity, one 1 cm × 1 cm sinus mucosal section was fixed in 2% formalin solution and sent for histopathology analysis (Adelaide Pathology and Partners, Adelaide, Australia). Samples were stained with hematoxylin & eosin and embedded in paraffin. Using light microscopy (Eclipse 90i, Nikon instruments Inc, Melville, NY, USA) a blinded pathologist performed microscopic evaluation and tissue grading. A Likert scale was used to grade, acute inflammation, oedema and fibrosis (Table 1).
Table 1
| Variable | Acute inflammation (0–2) | Oedema (0–3) | Fibrosis (0–3) |
|---|---|---|---|
| NT | |||
| M6 left | 1 | 2 | 0 |
| M6 right | 1 | 2 | 1 |
| M7 left | 1 | 2 | 0 |
| M7 right | 2 | 2 | 0 |
| M11 left | 1 | 2 | 1 |
| M11 right | 1 | 2 | 0 |
| M12 left | 1 | 2 | 0 |
| M12 right | 1 | 2 | 0 |
| M13 left | 1 | 2 | 0 |
| M13 right | 1 | 2 | 0 |
| CG | |||
| M1 left | 1 | 2 | 0 |
| M1 right | 1 | 1 | 0 |
| M3 left | 1 | 2 | 0 |
| M3 right | 2 | 2 | 0 |
| M5 left | 1 | 2 | 1 |
| M5 right | 1 | 2 | 1 |
| M8 left | 1 | 2 | 1 |
| M8 right | 1 | 2 | 0 |
| M14 left | 1 | 2 | 0 |
| M 14 right | 1 | 2 | 0 |
| CG-BM | |||
| M2 left | 1 | 2 | 0 |
| M2 right | 0 | 2 | 0 |
| M4 left | 2 | 2 | 1 |
| M4 right | 1 | 1 | 0 |
| M9 left | 1 | 1 | 0 |
| M9 right | 1 | 1 | 0 |
| M10 left | 1 | 2 | 0 |
| M10 right | 1 | 2 | 0 |
| M15 left | 1 | 2 | 0 |
| M15 right | 1 | 2 | 0 |
NT, no treatment; CG, chitogel; CG-BM, chitogel-budesonide-mupirocin.
Statistical analysis
Previous studies have described a 60% biofilm biomass reduction with treatment (14). To obtain a power of 80% and achieve a significance level of α=0.05 four sheep were required per arm. Five animals per arm were used in this study as this was our first experience investigating the efficacy of a topical gel treatment.
Bacterial biofilms were compared between all groups and analysed with 1-way analysis of variance (ANOVA) Dunnett’s multiple comparison test using GraphPad Prism 8.0 software (San Diego, CA, USA).
Results
Anti-inflammatory effects
There were no significant differences in acute inflammation, oedema and fibrosis between sinus mucosa treated with NT and CG-BM (Table 1, Figure 2).
Antibiofilm effects
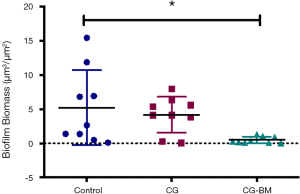
There was a significant reduction in biofilm biomass of CG-BM treated sheep compared to NT controls [P=0.01, 4.74 (95% CI: 0.85 to 8.6)]. There were no significant difference between NT and CG treated sheep or CG and CG-BM treated sheep (Figure 3).
Discussion
In this study we demonstrated that CG-BM significantly reduced S. aureus biofilms in an in vivo sinusitis model. Compared to no-treatment controls, CG-BM and CG reduced S. aureus biofilms by 90.5% and 20% respectively.
CG comprises succinyl-chitosan which is a chitosan polymer produced by the hydrolysis of chitin, found in the exoskeletons of crustaceans. In the last decade, CG has been largely used in ENT surgery to improve patient’s outcome post endoscopic sinus surgery (16-19) due to its effective hemostatic (18-26), wound healing (27-29), anti-adhesion (17,30-38) and antimicrobial (39-41) properties. However, its role as a drug release vehicle delivering topical treatments into sinus cavities has yet to be explored.
We hypothesize that by incorporating topical antibiotics into CG we are able to eradicate biofilms by direct application of antibiotic gel into sinuses, increase mucosal contact time with topical agents and use higher concentrations of antibiotics at target sites with less systemic side effects. Previous studies have also suggested that chitosan enhances the nasal absorption of topical treatments by the bioadhesivity of the polymer to mucosa and a transient widening of the nasal mucosa tight junctions (42,43).
Mupirocin was chosen to be the antimicrobial of choice to be incorporated into CG due to its known clinical efficacy as a nasal irrigation for patients with recalcitrant S. aureus CRS (44-48). Jervis-Bardy et al reported 88.9% of surgically recalcitrant patients had eradication of S. aureus using twice daily nasal lavages containing 0.05% mupirocin for 3 weeks (48). Mupirocin exerts its antimicrobial activity by irreversibly binding to the bacterial enzyme isoleucyl-transfer RNA, thereby preventing isoleucine incorporation during bacterial synthesis (49-51). It has excellent activity against staphylococci including MRSA, most streptococci, and against certain gram negative bacteria including Haemophilus influenzae (50) which are increasingly prevalent in clinical practice.
Budesonide irrigations are now widely recognised to be safe (52-54) and effective in improving symptoms and endoscopic outcomes post sinus surgery (55-57). Luo et al. suggested that hydrophobic drugs like steroids are released more slowly from hydrogels (58), making CG an excellent controlled release vehicle for budesonide. Our department has recently demonstrated that by incorporating budesonide in CG applied post sinus surgery, there was endoscopic evidence of reduced inflammation during the early postoperative period and reduction in the extent of ostial stenosis at 3 and 12 months (15) suggesting possible anti-inflammatory and anti-adhesion properties. CG-BM treated mucosa showed a trend to improvement in acute inflammation, oedema and fibrosis when compared to saline rinses, but was not statistically significant. Therefore we did not further our investigation on anti-inflammatory effects of individual gel components
We postulate that this could be a result of several different factors. Firstly, only intermediate levels of inflammation were observed in our positive control sheep, indicating that S. aureus biofilms might not induce massive inflammation in sheep as would normally be expected. This could be due to the fact that sheep sinus mucosa is naturally colonised by a range of bacteria and might be adapted to the presence of biofilms already. Secondly, the release of budesonide from CG was found to be only up to 18% of the total possible amount over 72 hours (unpublished observations). Optimising the pharmacological formulation of budesonide for improved release from CG might help to improve the anti-inflammatory effects of CG-Bud gel. Also, it is possible that the self-limiting inflammatory process post-surgery is different to inflammation secondary to a bacterial nidus, which might require a higher concentration or longer exposure of budesonide for an increased effect.
CG-BM gel can be applied in the outpatient setting under direct endoscopic view into post-ESS infected sinus cavities via curved and straight suction cannulas. This has the theoretical benefit of effective anti-biofilm delivery specific to infected sinuses regardless of ostium size, patient douching technique and compliance. However further clinical studies are required to explore this treatment viability.
This study has several limitations, one such limitation was our inability to quantify the amount of treatment gel remaining in each sinus over the treatment course due to differing sinus anatomy or drainage. Therefore, it is possible that each sinus was exposed to variable drug concentrations during treatment. Another limitation of this study remains that in vivo studies cannot fully simulate the conditions of human sinuses and clinical studies will be required to fully characterise the potential of this treatment.
Conclusions
Our study concludes that CG-BM significantly reduces S. aureus biofilms in a sheep sinusitis model. The use of CG as a means to deliver topical therapies offers otolaryngologists a potential alternative to manage surgically recalcitrant CRS.
Acknowledgments
We thank Loren Matthews, Robb Muirhead, Paul Herde, Kevin Neuman, Dr Tim Kuchel and Carol Hewitt for their exceptional technical support at the Large Animal Research and Imaging Facility (LARIF). We also thank Lincoln Yardley from the Murray Bridge Abattoir for the supply of sheep heads for our optimisation study.
Funding: Sources came from the University of Adelaide, School of Medicine, Department of Otolaryngology Head and Neck Surgery, Adelaide, South Australia, Australia.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-18-97). AJP serves as an unpaid editorial board member of Australian Journal of Otolaryngology and Consultant for Medtronic, ENT technologies, Tissium. SM reports other from Chitogel, during the conduct of the study; other from Chitogel, outside the submitted work; in addition, SM has a patent Chitogel issued. PJW reports other from Chitogel, during the conduct of the study; other from Chitogel, outside the submitted work; in addition, PJW has a patent Chitogel issued. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Animal Ethics Committee of The University of Adelaide and the South Australian Health and Medical Research Institute (SAHMRI). All reports on animal experiments have been conducted in accordance with institutional and national guidelines for the care and use of laboratory animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singhal D, Foreman A, Jervis-Bardy J, et al. Staphylococcus aureus biofilms: Nemesis of endoscopic sinus surgery. Laryngoscope 2011;121:1578-83. [Crossref] [PubMed]
- Singhal D, Psaltis AJ, Foreman A, et al. The impact of biofilms on outcomes after endoscopic sinus surgery. Am J Rhinol Allergy 2010;24:169-74. [Crossref] [PubMed]
- Psaltis AJ, Weitzel EK, Ha KR, et al. The effect of bacterial biofilms on post-sinus surgical outcomes. Am J Rhinol 2008;22:1-6. [Crossref] [PubMed]
- Prince AA, Steiger JD, Khalid AN, et al. Prevalence of biofilm-forming bacteria in chronic rhinosinusitis. Am J Rhinol 2008;22:239-45. [Crossref] [PubMed]
- Bendouah Z, Barbeau J, Hamad WA, et al. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg 2006;134:991-6. [Crossref] [PubMed]
- Foreman A, Psaltis AJ, Tan LW, et al. Characterization of bacterial and fungal biofilms in chronic rhinosinusitis. Am J Rhinol Allergy 2009;23:556-61. [PubMed]
- Stephenson MF, Mfuna L, Dowd SE, et al. Molecular characterization of the polymicrobial flora in chronic rhinosinusitis. J Otolaryngol Head Neck Surg 2010;39:182-7. [PubMed]
- Costerton JW. Overview of microbial biofilms. J Ind Microbiol 1995;15:137-40. [Crossref] [PubMed]
- Harvey RJ, Schlosser RJ. Local drug delivery. Otolaryngol Clin North Am 2009;42:829-45. ix. [Crossref] [PubMed]
- Liang J, Lane AP. Topical Drug Delivery for Chronic Rhinosinusitis. Curr Otorhinolaryngol Rep 2013;1:51-60. [Crossref] [PubMed]
- Beule A, Athanasiadis T, Athanasiadis E, et al. Efficacy of different techniques of sinonasal irrigation after modified Lothrop procedure. Am J Rhinol Allergy 2009;23:85-90. [Crossref] [PubMed]
- Grobler A, Weitzel EK, Buele A, et al. Pre- and postoperative sinus penetration of nasal irrigation. Laryngoscope 2008;118:2078-81. [Crossref] [PubMed]
- Ha KR, Psaltis AJ, Tan L, et al. A sheep model for the study of biofilms in rhinosinusitis. Am J Rhinol 2007;21:339-45. [Crossref] [PubMed]
- Le T, Psaltis A, Tan LW, et al. The efficacy of topical antibiofilm agents in a sheep model of rhinosinusitis. Am J Rhinol 2008;22:560-7. [Crossref] [PubMed]
- Ha T, Valentine R, Moratti S, et al. The efficacy of a novel budesonide chitosan gel on wound healing following endoscopic sinus surgery. Int Forum Allergy Rhinol 2018;8:435-43. [Crossref] [PubMed]
- Ngoc Ha T, Valentine R, Moratti S, et al. A blinded randomized controlled trial evaluating the efficacy of chitosan gel on ostial stenosis following endoscopic sinus surgery. Int Forum Allergy Rhinol 2013;3:573-80. [Crossref] [PubMed]
- Athanasiadis T, Beule AG, Robinson BH, et al. Effects of a novel chitosan gel on mucosal wound healing following endoscopic sinus surgery in a sheep model of chronic rhinosinusitis. Laryngoscope 2008;118:1088-94. [Crossref] [PubMed]
- Chung YJ, An SY, Yeon JY, et al. Effect of a Chitosan Gel on Hemostasis and Prevention of Adhesion After Endoscopic Sinus Surgery. Clin Exp Otorhinolaryngol 2016;9:143-9. [Crossref] [PubMed]
- Valentine R, Athanasiadis T, Moratti S, et al. The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery. Am J Rhinol Allergy 2010;24:70-5. [Crossref] [PubMed]
- Chou TC, Fu E, Wu CJ, et al. Chitosan enhances platelet adhesion and aggregation. Biochem Biophys Res Commun 2003;302:480-3. [Crossref] [PubMed]
- Klokkevold PR, Lew DS, Ellis DG, et al. Effect of chitosan on lingual hemostasis in rabbits. J Oral Maxillofac Surg 1991;49:858-63. [Crossref] [PubMed]
- Klokkevold PR, Subar P, Fukayama H, et al. Effect of chitosan on lingual hemostasis in rabbits with platelet dysfunction induced by epoprostenol. J Oral Maxillofac Surg 1992;50:41-5. [Crossref] [PubMed]
- Pusateri AE, McCarthy SJ, Gregory KW, et al. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Trauma 2003;54:177-82. [Crossref] [PubMed]
- Rao SB, Sharma CP. Use of chitosan as a biomaterial: studies on its safety and hemostatic potential. J Biomed Mater Res 1997;34:21-8. [Crossref] [PubMed]
- Valentine R, Athanasiadis T, Moratti S, et al. The efficacy of a novel chitosan gel on hemostasis after endoscopic sinus surgery in a sheep model of chronic rhinosinusitis. Am J Rhinol Allergy 2009;23:71-5. [Crossref] [PubMed]
- Valentine R, Boase S, Jervis-Bardy J, et al. The efficacy of hemostatic techniques in the sheep model of carotid artery injury. Int Forum Allergy Rhinol 2011;1:118-22. [Crossref] [PubMed]
- Biagini G, Bertani A, Muzzarelli R, et al. Wound management with N-carboxybutyl chitosan. Biomaterials 1991;12:281-6. [Crossref] [PubMed]
- Stone CA, Wright H, Clarke T, et al. Healing at skin graft donor sites dressed with chitosan. Br J Plast Surg 2000;53:601-6. [Crossref] [PubMed]
- Azad AK, Sermsintham N, Chandrkrachang S, et al. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res B Appl Biomater 2004;69:216-22. [Crossref] [PubMed]
- Costain DJ, Kennedy R, Ciona C, et al. Prevention of postsurgical adhesions with N,O-carboxymethyl chitosan: examination of the most efficacious preparation and the effect of N,O-carboxymethyl chitosan on postsurgical healing. Surgery 1997;121:314-9. [Crossref] [PubMed]
- Diamond MP, Luciano A, Johns DA, et al. Reduction of postoperative adhesions by N,O-carboxymethylchitosan: a pilot study. Fertil Steril 2003;80:631-6. [Crossref] [PubMed]
- Kennedy R, Costain DJ, McAlister VC, et al. Prevention of experimental postoperative peritoneal adhesions by N,O-carboxymethyl chitosan. Surgery 1996;120:866-70. [Crossref] [PubMed]
- Cabral JD, McConnell MA, Fitzpatrick C, et al. Characterization of the in vivo host response to a bi-labeled chitosan-dextran based hydrogel for postsurgical adhesion prevention. J Biomed Mater Res A 2015;103:2611-20. [Crossref] [PubMed]
- Medina JG, Das S. Sprayable chitosan/starch-based sealant reduces adhesion formation in a sheep model for chronic sinusitis. Laryngoscope 2013;123:42-7. [Crossref] [PubMed]
- Medina JG, Steinke JW, Das S. A chitosan-based sinus sealant for reduction of adhesion formation in rabbit and sheep models. Otolaryngol Head Neck Surg 2012;147:357-63. [Crossref] [PubMed]
- Vlahos A, Yu P, Lucas CE, et al. Effect of a composite membrane of chitosan and poloxamer gel on postoperative adhesive interactions. Am Surg 2001;67:15-21. [PubMed]
- Zhou J, Elson C, Lee TD. Reduction in postoperative adhesion formation and re-formation after an abdominal operation with the use of N, O - carboxymethyl chitosan. Surgery 2004;135:307-12. [Crossref] [PubMed]
- Zhou J, Lee JM, Jiang P, et al. Reduction in postsurgical adhesion formation after cardiac surgery by application of N,O-carboxymethyl chitosan. J Thorac Cardiovasc Surg 2010;140:801-6. [Crossref] [PubMed]
- Paramasivan S, Jones D, Baker L, et al. The use of chitosan-dextran gel shows anti-inflammatory, antibiofilm, and antiproliferative properties in fibroblast cell culture. Am J Rhinol Allergy 2014;28:361-5. [Crossref] [PubMed]
- No HK, Park NY, Lee SH, et al. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 2002;74:65-72. [Crossref] [PubMed]
- Rhoades J, Roller S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl Environ Microbiol 2000;66:80-6. [Crossref] [PubMed]
- Illum L, Farraj NF, Davis SS. Chitosan as a novel nasal delivery system for peptide drugs. Pharm Res 1994;11:1186-9. [Crossref] [PubMed]
- Nakamura K, Maitani Y, Lowman AM, et al. Uptake and release of budesonide from mucoadhesive, pH-sensitive copolymers and their application to nasal delivery. J Control Release 1999;61:329-35. [Crossref] [PubMed]
- Seiberling KA, Aruni W, Kim S, et al. The effect of intraoperative mupirocin irrigation on Staphylococcus aureus within the maxillary sinus. Int Forum Allergy Rhinol 2013;3:94-8. [Crossref] [PubMed]
- Solares CA, Batra PS, Hall GS, et al. Treatment of chronic rhinosinusitis exacerbations due to methicillin-resistant Staphylococcus aureus with mupirocin irrigations. Am J Otolaryngol 2006;27:161-5. [Crossref] [PubMed]
- Uren B, Psaltis A, Wormald PJ. Nasal Lavage With Mupirocin for the Treatment of Surgically Recalcitrant Chronic Rhinosinusitis. Laryngoscope 2008;118:1677-80. [Crossref] [PubMed]
- Jervis-Bardy J, Wormald PJ. Microbiological outcomes following mupirocin nasal washes for symptomatic, Staphylococcus aureus-positive chronic rhinosinusitis following endoscopic sinus surgery. Int Forum Allergy Rhinol 2012;2:111-5. [Crossref] [PubMed]
- Jervis-Bardy J, Boase S, Psaltis A, et al. A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope 2012;122:2148-53. [Crossref] [PubMed]
- Boon RJ, Beale AS, Sutherland R. Efficacy of topical mupirocin against an experimental Staphylococcus aureus surgical wound infection. J Antimicrob Chemother 1985;16:519-26. [Crossref] [PubMed]
- Sutherland R, Boon RJ, Griffin KE, et al. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother 1985;27:495-8. [Crossref] [PubMed]
- Parenti MA, Hatfield SM, Leyden JJ. Mupirocin: a topical antibiotic with a unique structure and mechanism of action. Clin Pharm 1987;6:761-70. [PubMed]
- Welch KC, Thaler ER, Doghramji LL, et al. The effects of serum and urinary cortisol levels of topical intranasal irrigations with budesonide added to saline in patients with recurrent polyposis after endoscopic sinus surgery. Am J Rhinol Allergy 2010;24:26-8. [Crossref] [PubMed]
- Sachanandani NS, Piccirillo JF, Kramper MA, et al. The effect of nasally administered budesonide respules on adrenal cortex function in patients with chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg 2009;135:303-7. [Crossref] [PubMed]
- Bhalla RK, Payton K, Wright ED. Safety of budesonide in saline sinonasal irrigations in the management of chronic rhinosinusitis with polyposis: lack of significant adrenal suppression. J Otolaryngol Head Neck Surg 2008;37:821-5. [PubMed]
- Jang DW, Lachanas VA, Segel J, et al. Budesonide nasal irrigations in the postoperative management of chronic rhinosinusitis. Int Forum Allergy Rhinol 2013;3:708-11. [Crossref] [PubMed]
- Kang TW, Chung JH, Cho SH, et al. The Effectiveness of Budesonide Nasal Irrigation After Endoscopic Sinus Surgery in Chronic Rhinosinusitis With Asthma. Clin Exp Otorhinolaryngol 2017;10:91-6. [Crossref] [PubMed]
- Snidvongs K, Pratt E, Chin D, et al. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. Int Forum Allergy Rhinol 2012;2:415-21. [Crossref] [PubMed]
- Luo Y, Kirker KR, Prestwich GD. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release 2000;69:169-84. [Crossref] [PubMed]
Cite this article as: Ooi ML, Drilling AJ, James C, Moratti S, Vreugde S, Psaltis AJ, Wormald PJ. Efficacy of anti-biofilm gel, chitogel-mupirocin-budesonide in a sheep sinusitis model. Aust J Otolaryngol 2020;3:16.


