Desmoid tumours of the head and neck: an Australian case series
Introduction
Desmoid tumours (DT) (or aggressive fibromatosis) are benign fibroblastic tumours arising from muscle and fascia of deep soft tissues. Despite their benign nature and lack of metastatic potential, they are highly aggressive and have potential for invasion and destruction of surrounding tissues (1-3). Hence, their management is difficult and complex. These are rare tumours with an incidence of 2.4–4.5 cases per million annually, of which the majority arise intra-abdominally and only 12–15% occur in the head and neck (2-4). Head and neck desmoids are more likely to occur in the paediatric population (4).
A majority of DT (89%) are sporadic and associated with mutations in the β-catenin gene (somatic CTNNB1, T41A, S45F, S45P, S45N mutations), others are associated with germline APC gene mutations and Gardner syndrome (5). The pathological diagnosis of DT can be challenging and immunohistochemical staining for β-catenin is a useful tool in distinguishing DT from reactive myofibroblastic proliferation (5). The majority of APC gene mutation associated DT are intraabdominal and therefore uncommon in the head and neck. Given difficulty with histopathological diagnosis clinical features including growth rate and radiological findings are imperative in complementing pathological diagnosis and informing decision making for active treatment (6).
The clinical course of these tumours can be variable with spontaneous regression described in up to 27% of cases in all anatomical areas, hence a new push for active surveillance strategies with active treatment employed for pain or progression of disease (7). The rate of spontaneous remission remains unknown in the head and neck region.
When active treatment is initiated local control remains the ultimate goal of treatment. Consensus on optimal treatment modality in DT of the head and neck has not been reached. The intricate anatomy of the head and neck region makes achieving control of these tumours by surgery alone a challenge due to potential for significant side effects. Those that are not amenable to surgical resection due to significant morbidity often undergo primary chemotherapy or radiotherapy, the approach to which is varied in the literature. These tumours have a propensity for persistence and local recurrence (approximately 30%) and incomplete resection often requires adjuvant chemotherapy or radiotherapy (2).
Management of these tumours has been reported in small case series around the world. To date there are no Australian case series examining management of DT of the head and neck. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-20-38).
Methods
Study aims and objectives
This study aims to present an Australian experience in treating DT of the head and neck and to identify patient and disease features associated with event-free survival and mortality.
Study design
Retrospective multicentre case series.
Methodology
This study was undertaken as a collaboration between two centres in Sydney, Australia: Prince of Wales Hospital and Sydney Children’s Hospital, Randwick. The study was conducted in accordance with the Declaration of Helsinki (2013). Prior to commencement of the study ethics approval was sought and granted by the Human Research and Ethics Committee attached to South Eastern Sydney Local Health District (2019/ETH08774). Hospital medical records and oncology databases were searched for cases of DT in the head and neck region in the period 2000 to 2019 at both institutions. Participants were deemed suitable for inclusion if they had received a diagnosis of head and neck DTs by means of examination of histopathological specimens obtained at the time of surgical resection of the affected anatomical site, or on fine needle aspirate (FNAB)/core needle biopsy. Patients’ medical records were reviewed and demographic and clinical data including age, gender, date of pathological diagnosis, method of diagnosis (FNAB/core biopsy/operative sample), anatomic site of primary tumour, presenting symptoms and imaging modalities used for diagnosis [ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET)] were extracted. Imaging studies and notes from time of operation were used to define primary anatomical site of tumour.
Histopathological data was extracted from histopathology reports. Macroscopic and microscopic variables included presence of positive margins and invasion of local structures, including tissue type invaded. Other microscopic variables included perineural and vascular invasion.
Treatment related information was similarly extracted from patient medical records including details of surgery, radiotherapy and chemotherapy and evidence of persistence post-surgery.
Primary survival outcomes were event-free and overall survival. Event-free survival was defined as time from local control (surgical or otherwise) to date of recurrence, or time from diagnosis to treatment failure in those that did not achieve disease freedom from primary treatment. Overall survival was defined as time from pathological diagnosis to death. These data points were obtained by examining follow up clinic records and contacting patients’ general practitioners for survival information.
Statistical method
All statistical analysis was undertaken using IBM SPSS Statistics Version 25 (Armonk, New York, USA). Descriptive statistics were calculated. Survival analysis was undertaken by generating Kaplan-Meier curves to calculate event-free survival and overall survival for the entire cohort.
Results
Demographics and tumour location
Eight cases of DT of the head and neck in the period 2000 to 2019 were identified from both centres and included for analysis. There was a female predominance (62.5%), 5 patients were paediatric and 3 were adult cases. The overall mean and median age at diagnosis was 20.18±24.6 and 7.47 years, respectively. Within the paediatric cohort, 3 (60%) patients were female and the mean age at diagnosis was 3.6±4.6 years. The youngest case was diagnosed at ten months old. Within the adult population, 2 (66.7%) patients were female and the mean age at diagnosis was 47.8±15.9 years (Table 1). Of these 8 cases, 4 tumours were located in the soft tissues of the neck (50%), 1 in the parapharyngeal wall (12.5%), 1 in the parotid gland (12.5%), 1 in the maxillary sinus (12.5%) and 1 in the mandible (12.5%). All patients presented with single unilateral tumours (Table 2).
Table 1
| Demographic data | Adult cases (n=3) | Paediatric cases (n=5) |
|---|---|---|
| Age at diagnosis (years) | ||
| Mean ± SD | 47.8±15.9 | 3.6±4.6 |
| Median | 48.4 | 1.2 |
| Sex, n (%) | ||
| Male | 1 (33.3) | 2 (40.0) |
| Female | 2 (66.7) | 3 (60.0) |
Table 2
| Case No. | Age at diagnosis (years) | Site | Primary treatment modality | Primary resection (macroscopic) | Margin involvement (microscopic) | Follow up time (months) | Neo/adjuvant treatment | Disease persistence | Disease recurrence |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | Parotid gland | Radiotherapy | N/A | N/A | 9.6 months | Nil | Unknown | Unknown |
| 2 | 1 | Lateral neck extending from skull base to clavicle) | Chemotherapy: naproxen, methotrexate, vinblastine, sorafenib, imatinib | Incomplete | Positive margins | 2.1 years | Salvage surgery at another centre | Yes | No |
| 3 | 11 | Lateral neck level I–V involving parotid gland and ipsilateral thyroid gland | Surgical resection | Incomplete | Positive margins | 4.4 years | Adjuvant radiotherapy following salvage surgery | Yes | No |
| 4 | 48 | Parapharyngeal space | Surgical resection | Complete | Clear margins | 15.1 years | Radiotherapy | No | Yes (6 and 14 years) |
| 5 | 1 | Maxillary sinus | Surgical resection and free flap reconstruction | Complete | Positive margins | 10.0 years | Nil | No | No |
| 6 | 31 | Posterior neck (trapezius) | Radiotherapy | N/A | N/A | 4.0 years | Nil | No | No |
| 7 | 10 months | Parotid gland | Surgical resection | Complete | Positive margins | 3.1 years | Neoadjuvant vinblastine and methotrexate | No | No |
| 8 | 3 | Mandible | Surgical resection and free flap reconstruction | Complete | Positive margins | 1.2 years | Adjuvant radiotherapy | No | No |
Primary treatment modalities
Five out of eight patients underwent primary surgical resection of which four had either microscopic or macroscopic margin involvement. Of these patients, only one patient did not achieve local control and required salvage operative management. One patient received pre-operative chemotherapy with vinblastine and methotrexate and two patients received adjuvant radiotherapy. Two patients underwent primary radiotherapy and one patient received chemotherapy with naproxen, methotrexate, vinblastine, sorafenib and imatinib (Table 2).
Pathologic features of disease
Histopathological examination confirmed the diagnosis of DT in all 6 patients who underwent surgical resection. Of these, 5 tumours invaded local structures (83.3%). Tissues invaded included adipose (3, 50%), muscle (3, 50%), nerve (2, 33.3%), large blood vessel (2, 33.3%) and bone (2, 33.33%). Additionally, 3 tumours displayed perineural invasion (50%) and 2 tumours displayed microvascular (33.3%) invasion. Of the six patients who underwent surgical resection at any time, only 1 patient had clear microscopic margins (16.7%) (Table 3). Two patients who received non-surgical treatment as primary modality had diagnosis confirmed on fine needle aspirate or core biopsy and therefore these cases were not amenable to full histopathological analysis.
Table 3
| Histopathological feature | n (%) |
|---|---|
| Total specimens examined | 6 (100.0) |
| Positive margins | 5 (83.3) |
| Invasion adjacent tissue | 5 (83.3) |
| Bone | 2 (33.3) |
| Muscle | 3 (50.0) |
| Nerve | 2 (33.3) |
| Large vessel | 2 (33.3) |
| Adipose | 3 (50.0) |
| Microvascular invasion | 2 (33.3) |
| Perineural invasion | 3 (50.0) |
Survival outcomes
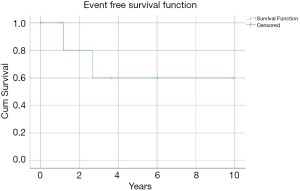
One patient was lost to follow up and as such seven patients were included in the survival analysis. Mean follow up time was 5.47±5.53 years (range, 0.79–15.12 years). Mean overall survival for the included cohort at last follow up was 5.09±4.97 years and 85.7% at final follow up. Mean event-free survival was 6.74±1.77 years and 71.4% for the entire cohort. Two patients failed to achieve local control after primary treatment and one patient experienced local disease recurrence at 3 and 13 years post primary treatment resulting in disease specific mortality at 15.1 years. Local control at time of final follow up was 71.4% (Figure 1). Subgroup survival analyses were not performed due to limited cohort numbers.
There were two cases in which disease persisted after primary treatment (Table 4). Both cases were paediatric patients. The first patient (10 months old at diagnosis) underwent primary chemotherapy (naproxen, methotrexate, vinblastine, imatinib and sorafenib) for a neck tumour which was seen to encase the left internal carotid artery on MRI. Repeat imaging after primary treatment demonstrated marked growth of the tumour. This patient subsequently underwent surgical resection at a different institution, requiring sacrifice of the left internal carotid artery and cranial nerves VII–XII. The patient consequently developed a unilateral vocal cord palsy requiring tracheostomy for multiple aspiration events. This was followed by chemotherapy with doxorubicin and nirogacestat with disease persistence ultimately ensuing at the end of treatment. This patient was subsequently palliated given a lack of response to treatment.
Table 4
| Case | Persistence/recurrence | Further treatment | Outcome |
|---|---|---|---|
| 2 | Persistence | Surgical Resection at different centre. Adjuvant doxorubicin and subsequent nirogacestat | Ongoing disease progression and failure to achieve local control with significant treatment and disease related morbidity† |
| 3 | Persistence | Pegylated liposomal doxorubicin without benefit. Subsequent secondary surgery with adjuvant radiotherapy | Local control |
| 4 | Recurrence at 3- and 13-year post completion of initial primary/adjuvant treatment | Repeat Radiotherapy at each recurrence. Tamoxifen and NSAID therapy at final recurrence | Progression of recurrent disease and disease related mortality at 15.1 years |
†, patient died from disease following reporting of data.
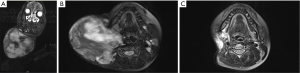
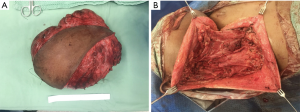
The second case of disease persistence occurred in a 16-year-old female patient who had undergone incomplete primary surgical resection with remaining macroscopic disease at an overseas facility (Table 4). After significant tumour growth, with preoperative images illustrating classic MRI features of DT, this patient subsequently underwent complete macroscopic resection (Figure 2) (8). This patient’s tumour measured 21 cm × 16 cm × 9 cm, intraoperative findings revealed the tumour extended superiorly abutting mandible, submandibular gland, and parotid tissue, inferiorly to clavicle, posteriorly to pre-vertebral fascia and medially to ipsilateral thyroid lobe (Figure 3). The tumour was adherent to internal carotid artery and required sacrifice of the spinal accessory nerve, sternocleidomastoid, internal and external jugular veins and branches of the cervical plexus. Hypoglossal and vagus nerves were able to be preserved. She underwent adjuvant radiotherapy for positive margins and demonstrated disease control and no persistence at final follow up.
There was one case of disease recurrence resulting in the only mortality of this series. This was an adult patient who had undergone primary surgical resection and adjuvant radiotherapy for a left parapharyngeal space tumour. Initial disease recurrence in the lower neck was noted 6 years later and was treated with radiotherapy. A subsequent recurrence occurred a further 8 years later which was treated with radiotherapy and tamoxifen/non-steroidal anti-inflammatory drugs (NSAIDs). Disease related mortality occurred 15.1 years from initial treatment (Table 4).
Discussion
There are varying reports on the demographic nature and clinicopathologic features of these tumours. This series demonstrated a female predilection both in the adult and paediatric population in keeping with some previous series (9-11) but not identified in other data (2).
There appears to be a propensity for paediatric cases in the literature for DT in the head and neck, a trend that is evident in our data. A recent review demonstrated a mean age of 16.87 years with over half of cases in patients <11 years of age (2).
In our population, both paediatric and adult cases presented variably in the anatomy involved. This series highlighted a predominance for tumours arising from soft tissue in the neck similar to that found in previous series (9-13). Of note, the only tumours arising from bone (mandible and maxilla) were in the paediatric population. Kruse et al. [2010] reported the mandible to be the most common site in a series with predominantly paediatric patients (2). Another series reported mandibular disease in 38% of paediatric cases (14). Bony involvement appears to be a risk factor for recurrence, the same series reporting 24% in those arising from bone (mandible 16%, maxillary 5% and cranial/ base of skull 3%) (2). The literature is varied on age <18 years predicting poorer outcomes, some studies illustrating poorer local control rates in paediatric cases (15) and others indicating age has no influence on recurrence rates (2,16).
The challenge of treating DT in the head and neck region remains the variability in the tissues they involve, lack of a tumour capsule, capacity for local progression, propensity to occur in young patients and morbidity associated with surgical resection.
Worldwide, treatment approaches to DT are highly varied, some centres implementing ‘watch and wait’ strategies given up to 27% of desmoids undergo spontaneous remission (7).
A recent large series examining DT in all anatomical areas from the French Sarcoma Group demonstrated no difference in event-free survival between primary surgical treatment and watch and wait approaches (17). Thus, current consensus guidelines recommend implementing active treatment only in cases of pain and disease progression (6).
Where active treatment is initiated, very low quality evidence suggests there is no difference between radiotherapy and surgery alone with regard to risk of progressive disease (6). There is very little high quality evidence to guide decision-making on the benefit of adjuvant radiotherapy compared to surgery alone (6). However, in the meta-analysis by Janssen et al. adjuvant radiotherapy improved recurrence rates in patients with incomplete but not clear surgical margins (18).
The role of surgical management specifically in head and neck DT has not been well established. Given the rarity of extra-abdominal DT presenting in the head and neck region, a paucity of evidence exists guiding optimum treatment approach. The majority of reports of these tumours exist in low level evidence small case series. Consensus guidelines identify head and neck desmoids as tumours in an unfavourable location in which event-free survival may be better in the watch and wait group for those who are not symptomatic of disease but this is limited by poor quality data (9).
A number of systemic medical therapies are available with varying levels of evidence supporting their use. National Comprehensive Cancer Network (NCCN) guidelines suggest systemic therapy as an option for unresectable DT and those for which surgery would be unacceptably morbid (19). These include anti-hormonal therapies (tamoxifen/toremifene) in combination with NSAIDs, and tyrosine kinase inhibitors (imatinib, nilotinib, sorafenib, pazopanib) which have been shown in multiple randomised trials to achieve favourable results in all anatomical sites with 1 year progression free survival of up to 67% (20). Low dose chemotherapy regimens including methotrexate plus vinblastine/vinorelbine, anthracycline based regimens and pegylated liposomal doxorubicin have also been shown to achieve progression free survival rates of up to 88% at 12 months (6,21). Cytotoxic chemotherapeutic agents are increasingly reserved for urgent and severely symptomatic cases in light of their long-term side-effects and non-cytotoxic agents (tamoxifen/ NSAIDs) are being employed more and more as first line therapy where surgical resection is not indicated (19).
In this series we describe excellent local control outcomes in patients appropriately selected for surgical management (71.4% after primary treatment). This is especially pertinent in paediatric cases given the long-term complications of radiotherapy and chemotherapy.
Surgery for DT can carry significant morbidity in the head and neck region. Sacrifice of nerves, blood vessels, muscle and bone may be required for adequate local control of disease and incomplete resection increases the risk of recurrence by two-fold in those with positive margins (18).
Previous literature indicates negative microscopic surgical margins are uncommon, rates varying from 38-55%. Negative microscopic margins were achieved in 16.67% in our series, however despite this, overall survival and local control occurred at rates of 87.5% and 71.4% indicating that, contrary to the literature, surgical margins may not primarily influence disease outcomes when patients are appropriately selected for surgery (10,11,13).
The single case of disease recurrence in this series achieved negative surgical margins at primary resection. While some studies have demonstrated a beneficial relationship between negative margins and recurrence in the paediatric population (22-24), a number of other series have shown no relationship, with no additional benefit from wide surgical margins in comparison to margins <1 mm (25-27).
Beta catenin and p53 staining remains an important consideration in prognosticating recurrence, evidence suggesting that overexpression is linked with decreased event-free survival in DT in all anatomical areas (28), others suggesting that in the head and neck, expression of beta catenin should not be considered a specific marker for aggressive behaviour of the tumours (29).
Nirogacestat is a novel agent that has recently gained FDA approval for investigation in a phase III clinical trial for the treatment of progressive, unresectable, recurrent or refractory DT (30-32). The proposed mechanism is blocking of gamma-secretase which is thought to be implicated in tumour cell growth (33). Increasingly, advances in our understanding of the molecular biology of DT will guide ‘precision medicine’ and new therapeutic targets in the treatment of this disease (34,35).
This study is limited by small numbers and short follow up. Thus, these results should be interpreted as short term outcomes and initiation of active treatment such as surgery should be discussed at Head and Neck multidisciplinary meetings by experienced clinicians to make individualised recommendations. Key considerations in the management of head and neck DT are indications for active treatment (such as pain and disease progression in those being surveilled), morbidity associated with surgery (including requirement for sacrifice of cranial nerves and/or major blood vessels), quality of life considerations, long term sequelae of chemotherapy and radiotherapy in paediatric patients, and the evolving landscape of systemic treatments guided by advances in molecular understanding of these rare tumours.
Conclusions
In appropriately selected patients with head and neck DT, it is possible to achieve local control with surgery alone. Adjuvant radiotherapy may provide extra benefit in the setting of positive surgical margins. Where surgical resection is anticipated to cause significant functional and cosmetic morbidity, other treatment modalities, such as radiotherapy or systemic therapy, should be considered. The management of this disease is complex and therefore requires experienced clinicians in a multi-disciplinary setting to make individualised recommendations on optimum therapy.
Acknowledgments
Funding: The Head and Neck Cancer Foundation, Sydney, Australia (www.headandneckfoundation.com.au) for their generous support in funding this research.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-20-38
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-20-38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (2013). The study was approved by the South Eastern Sydney Local Health District human research ethics committee (2019/ETH08774) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO Classification of Tumours of Soft Tissue and Bone 2013.
- Kruse AL, Luebbers HT, Gratz KW, et al. Aggressive fibromatosis of the head and neck: a new classification based on a literature review over 40 years (1968-2008). Oral Maxillofac Surg 2010;14:227-32. [Crossref] [PubMed]
- Ballo MT, Zagars GK, Pollack A, et al. Desmoid tumor: Prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol 1999;17:158-67. [Crossref] [PubMed]
- Alherabi AZ, Marglani OA, Bukhari DH, et al. Desmoid tumor (fibromatosis) of the head and neck. Saudi Med J 2015;36:101-3. [Crossref] [PubMed]
- Trautmann M, Rehkämper J, Gevensleben H, et al. Novel pathogenic alterations in pediatric and adult desmoid-type fibromatosis - A systematic analysis of 204 cases. Sci Rep 2020;10:3368. [Crossref] [PubMed]
- Desmoid Tumor Working Group. The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer 2020;127:96-107. [Crossref] [PubMed]
- van Houdt WJ, Husson O, Patel A, et al. Outcome of Primary Desmoid Tumors at All Anatomic Locations Initially Managed with Active Surveillance. Ann Surg Oncol 2019;26:4699-706. [Crossref] [PubMed]
- Kamali F, Wang WL, Guadagnolo BA, et al. MRI may be used as a prognostic indicator in patients with extra-abdominal desmoid tumours. Br J Radiol 2016;89:20150308. [Crossref] [PubMed]
- Masson JK, Soule EH. Desmoid tumors of the head and neck. Am J Surg 1966;112:615-22. [Crossref] [PubMed]
- Hoos A, Lewis JJ, Urist MJ, et al. Desmoid tumors of the head and neck--a clinical study of a rare entity. Head & Neck 2000;22:814-21. [Crossref] [PubMed]
- Wang CP, Chang YL, Ko JY, et al. Desmoid tumor of the head and neck. Head & Neck 2006;28:1008-13. [Crossref] [PubMed]
- Fasching MC, Saleh J, Woods JE. Desmoid tumors of the head and neck. Am J Surg 1988;156:327-31. [Crossref] [PubMed]
- Conley J, Healey WV, Stout AP. Fibromatosis of the head and neck. Am J Surg 1966;112:609-14. [Crossref] [PubMed]
- Peña S, Brickman T, StHilaire H, et al. Aggressive fibromatosis of the head and neck in the pediatric population. Int J Pediatr Otorhinolaryngol 2014;78:1-4. [Crossref] [PubMed]
- Spear MA, Jennings LC, Mankin HJ, et al. Individualizing management of aggressive fibromatoses. Int J Radiat Oncol Biol Phys 1998;40:637-45. [Crossref] [PubMed]
- Scougall P, Staheli LT, Chew DE, et al. Desmoid tumors in childhood. Orthop Rev 1987;16:481-8. [PubMed]
- Penel N, Le Cesne A, Bonvalot S, et al. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: A nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer 2017;83:125-31. [Crossref] [PubMed]
- Janssen ML, van Broekhoven DL, Cates JM, et al. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br J Surg 2017;104:347-57. [Crossref] [PubMed]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma 2020 [updated 28/05/2020. 2]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- Penel N, Le Cesne A, Bui BN, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): An FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol 2011;22:452-7. [Crossref] [PubMed]
- Mir O, Rahal C, Rimareix F. Efficacy of oral vinorelbine in advanced/progressive desmoid tumours: an updated retrospective study in 50 patients. J Clin Oncol 2016;34:abstr 11050.
- Lakhan SE, Eager RM, Harle L. Aggressive juvenile fibromatosis of the paranasal sinuses: case report and brief review. J Hematol Oncol 2008;1:3. [Crossref] [PubMed]
- Faulkner LB, Hajdu SI, Kher U, et al. Pediatric desmoid tumor: retrospective analysis of 63 cases. J Clin Oncol 1995;13:2813-8. [Crossref] [PubMed]
- Buitendijk S, van de Ven CP, Dumans TG, et al. Pediatric aggressive fibromatosis: a retrospective analysis of 13 patients and review of literature. Cancer 2005;104:1090-9. [Crossref] [PubMed]
- Reitamo JJ. The desmoid tumor. IV. Choice of treatment, results, and complications. Arch Surg 1983;118:1318-22. [Crossref] [PubMed]
- Gronchi A, Casali PG, Mariani L, et al. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol 2003;21:1390-7. [Crossref] [PubMed]
- de Bree E, Zoras O, Hunt JL, et al. Desmoid tumors of the head and neck: a therapeutic challenge. Head & Neck 2014;36:1517-26. [PubMed]
- Gebert C, Hardes J, Kersting C, et al. Expression of beta-catenin and p53 are prognostic factors in deep aggressive fibromatosis. Histopathology 2007;50:491-7. [Crossref] [PubMed]
- Sharma A, Ngan BY, Sandor GK, et al. Pediatric aggressive fibromatosis of the head and neck: a 20-year retrospective review. J Pediatr Surg 2008;43:1596-604. [Crossref] [PubMed]
- Kummar S, O'Sullivan Coyne G, Do KT, et al. Clinical Activity of the γ-Secretase Inhibitor PF-03084014 in Adults With Desmoid Tumors (Aggressive Fibromatosis). J Clin Oncol 2017;35:1561-9. [Crossref] [PubMed]
- Children's Oncology Group ST, Inc. A Study of a New Drug, Nirogacestat, for Treating Desmoid Tumors That Cannot be Removed by Surgery: Clinical Trials Identifier NCT04195399: US National Library of Medicine: Clinical Trials.gov; 2019 [updated 14/05/2020]. Available online: https://clinicaltrials.gov/ct2/show/NCT04195399
- Villalobos VM, Hall F, Jimeno A, et al. Long-Term Follow-Up of Desmoid Fibromatosis Treated with PF-03084014, an Oral Gamma Secretase Inhibitor. Ann Surg Oncol 2018;25:768-75. [Crossref] [PubMed]
- Moore G, Annett S, McClements L, et al. Top Notch Targeting Strategies in Cancer: A Detailed Overview of Recent Insights and Current Perspectives. Cells 2020;9:1503. [Crossref] [PubMed]
- Shang H, Braggio D, Lee YJ, et al. Targeting the Notch pathway: A potential therapeutic approach for desmoid tumors. Cancer 2015;121:4088-96. [Crossref] [PubMed]
- Katoh M, Katoh M. Precision medicine for human cancers with Notch signaling dysregulation Int J Mol Med 2020;45:279-97. (Review). [PubMed]
Cite this article as: Sideris A, Fuzi J, Jacobson I, Wong W, Havas T. Desmoid tumours of the head and neck: an Australian case series. Aust J Otolaryngol 2020;3:32.