Computer-aided design and 3D printing for airway graft carving simulation and the effect on participants performance and confidence
Introduction
Surgical training relies heavily on practical experience. However, it can prove challenging for trainees to achieve proficiency and competency when exposure to certain cases and skill-sets are limited (1). This is especially relevant with regards to laryngeal and airway reconstruction surgery (2), given it is a relatively low frequency operation, with high stakes, advanced techniques, and unique equipment (1). Simulation is an effective means for trainees to develop these skills by allowing a safe, low-risk environment (2,3). Surgical simulation permits task achievement and acquisition of knowledge that can be transferrable to patient care, but without the ‘real-world’ consequences (3). While simulation models of varying materials and complexity have been investigated in current surgical literature, three-dimensional (3D) printing in particular has gained popularity for development of high-fidelity, low-cost, anatomically-accurate models, as it has become more accessible and available (3,4). 3D models generated by computer-aided design (CAD) have been utilized in many sub-specialty areas of otorhinolaryngology training (5-9). This technology holds advantage over many other types of simulation platforms as it allows visuospatial and tactile components of a surgical procedure to be reproduced (9,10). 3D printing was used in a recent study by Ha et al. [2017] to create a simulation tool for airway graft carving used in laryngotracheal reconstruction (LTR) (11). Similarly, this study aims to examine the usefulness and value of such a tool to improve trainees’ proficiency and confidence in airway reconstruction techniques.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-20-54).
Methods
The simulation training took place over one year during the Ear, Nose and Throat (ENT) Sinus Surgery Workshop in Perth, Western Australia (WA), the Australian Society of Otolaryngology Head and Neck Surgery (ASOHNS) annual meeting in Perth, and the WA ENT trainees breakfast education session.
Production of simulation tool
Using segmentation of a computed tomography (CT) scan of a normal 8-year-old pediatric rib, a standardized 3D-printed representation of a harvested human costal cartilage graft was created for this simulation, as per previously published methods (5,11). Previous studies have also validated the composition of the material (5,11).
Recruitment
All attendees, including consultant otolaryngologists, surgical education and training (SET) and non-SET registrars, fellows, and medical students, were invited to participate. Participants viewed a short instructional videos used in a previous pilot study, prior to the simulation (11).
Simulation and assessment
All participants who completed sequential simulations of carving an anterior and a posterior graft for airway reconstruction were included in this study. After completion, participants completed a previously-validated Likert scale survey which was used in an earlier study, and modified to question the participant confidence and ability (Figure 1) (11,12). The time taken to perform each graft was also measured and recorded. The final graft was assessed by the senior airway surgeon, and was blinded to participant level of training. A scale of 0 to 4 (poor, somewhat acceptable, average, excellent and superior) was used by the assessor to grade each graft.
Data analysis
Data was analyzed with the software program SPSS Statistics (SPSS Inc.). Chi square test, independent sample student’s t-test and paired sample student’s t-test were used. A P value of <0.05 was considered statistically significant.
The study did not require ethics application as no patients were involved, and consent was obtained from participants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Demographics
Twenty-seven participants who completed at least two anterior and/or posterior rib grafts were included in the analysis. Regarding anterior rib grafts, 25 participants completed two grafts, one completed only one graft, and one did not complete any anterior rib grafts. Regarding posterior rib grafts, 16 participants completed two grafts, one completed only one graft, while 9 did not complete any posterior rib grafts.
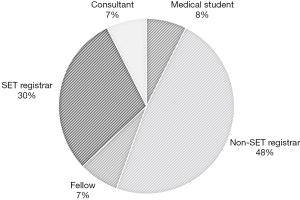
Two participants were qualified Otolaryngologists, 2 were fellows, 8 were SET trainees, 13 were non-SET trainees, and 2 were medical students (Figure 2). Almost half of participants (n=13, 48.1%) were more than 5 years from medical school graduation (post-graduate year: PGY). Majority (n=18, 66.7%) had not participated in any laryngotracheal reconstruction (LTR) procedures or cartilage grafting.
Time taken to graft
For both the anterior and posterior grafts, there was a significant difference observed between the mean time taken to carve the first and second graft—with the average first and second anterior grafts taking 6.13 and 4.78 min (P<0.01), respectively, and the first and second posterior grafts taking 5.23 and 3.51 min (P=0.02), respectively.
Confidence and ability
Participants were asked to rate their confidence after their first and second grafts. After performing one anterior rib grafting procedure, only 8 participants (29.6%) felt they had adequate confidence. After the second graft, this increased to 12 participants feeling adequately confident (44.4%) and a further 3 (11.1%) felt that they were very confident.
As for the posterior grafting procedure, only 3 (11.1%) felt they had adequate confidence after the first graft, but after the second graft, this increased to 7 participants (25.9%), while a further 2 participants (7.4%) felt very confident.
There was statistical significance between the self-rated ability between the first and second anterior grafts with a mean score increase from 2.09 to 2.65 (P=0.002). Similarly, with the first and second posterior grafts, there was a mean score increase from 1.71 to 2.57 (P<0.001). Those who had not participated in LTR rated their ability as improved on the second posterior rib graft (P=0.01).
Assessment of final product by senior airway surgeon
The final graft was assessed by the senior airway surgeon GEG. There were 26 participants who completed two anterior grafts and 16 who completed 2 posterior grafts. The mean score for the first anterior rib graft carving was 2.42, and the second one improved to 3.04 (P=0.002, CI: −0.84 to −0.39); the first posterior rib graft carving was 2.61, and the second one improved to 3.41 (P<0.001, CI: −1.16 to −0.53).
There was no significant association between the participants scores and their training level, whether they have had previous LTR or cartilage grafts experience. Nor did their previous cartilage graft carving experience significantly influence the timing of the simulated procedures.
Discussion
Surgical simulation allows for adequate opportunities to refine skills, achieve clinical competency, and importantly can positively impact patient outcomes and safety (1,13,14). In our experience, the 3D-printed costal cartilage grafting tool provided participants an opportunity to practice a skill-set used in LTR, as well as demonstrated an improvement in both skill and confidence.
In our study, there was significant improvement in average time taken to carve between the first and second grafts of both the anterior and posterior techniques, suggesting construct validity. Similarly, in a study using a 3D-printed simulation model, albeit for endoscopic ear surgery, Barber et al. [2016] showed that trainees were able to improve their performance time with more practice (6). Given that the duration of a surgery can often be longer with less-experienced trainees, the ability to reduce operative timing not only demonstrates skill familiarity and proficiency, but with it can provide the potential benefits of decreased theatre costs and anaesthetic safety of patients (15-17).
Participants were asked to rate their confidence after their first and second grafts. For both anterior and posterior graft, a greater number reported self-confidence after the second graft. Although confidence may not always translate into ability, it does allow exposure to a skill that can stimulate readiness to learn (9), and provides trainees with self-assurance for future procedures (18). In a study by Schwartz et al. [2018] assessing simulated paediatric airway procedures, the subjects’ confidence scores were assessed both pre- and post-simulation (19). Although most participants in our study had never participated in LTR, there did exist a varying level of experience. Therefore, in retrospect, it could have been valuable to apply the analysis used by Schwartz et al. [2018], in order to truly measure participant confidence improvement, without the association of surgical experience.
The self-rated ability of participants between the first and second grafts showed a significant increase in mean score. However, we also included an objective assessment by a senior airway surgeon, which confirmed that there was a significant improvement in the participants first and subsequent anterior and/or posterior graft. This helps further substantiate that participants were indeed able to learn the skills and consequently improve technically.
Comparable to the United States pilot study by Ha et al. [2017] on CAD and 3D printing for costal cartilage simulation (11), we too demonstrated that participant training level—whether they had previous LTR or cartilage grafts experience, did not affect scores nor timing.
Our study was limited by the small sample size. Although, this was not unexpected with the relative low number of available ENT service and trainee registrars and consultants, it would be useful for future studies to include a larger sample size, perhaps across states, to better validate outcomes. There was also a lack of longitudinal data or follow-up, which would be able to verify the longevity and transferability of the skills acquired. Additionally, a Likert questionnaire, while a simple and appropriate format to measure a scale-based task, can carry potential source for response bias. Given the format of the questionnaire, participants may be prompted to overvalue the simulator. This study is also limited by the uncertainty of how missing data from unanswered questionnaires may have affected the validity of results.
In conclusion, this simulation enabled participants of varying surgical experiences to improve relevant LTR skills both technically (anatomy, costal cartilage carving, instrumental use, equipment exposure) and cognitively (confidence and knowledge acquisition). With its advancing technology, 3D printing can easily fabricate a feasible, realistic, patient-specific simulation model, and is therefore a valuable tool for many areas within surgical education.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-20-54
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ajo-20-54
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-20-54). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study did not require ethics application as no patients were involved, and consent was obtained from participants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Myer CM 4th, Jabbour N. Advanced Pediatric Airway Simulation. Otolaryngol Clin North Am 2017;50:923-31. [Crossref] [PubMed]
- Burns JA, Adkins LK, Dailey S, et al. Simulators for Laryngeal and Airway Surgery. Otolaryngol Clin North Am 2017;50:903-22. [Crossref] [PubMed]
- Alwani M, Bandali E, Larsen M, et al. Current state of surgical simulation training in otolaryngology: systematic review of simulation training models. Archives of Otorhinolaryngology-Head & Neck Surgery 2019;3:5. [Crossref]
- VanKoevering KK, Malloy KM. Emerging Role of Three-Dimensional Printing in Simulation in Otolaryngology. Otolaryngol Clin North Am 2017;50:947-58. [Crossref] [PubMed]
- Berens AM, Newman S, Bhrany AD, et al. Computer-Aided Design and 3D Printing to Produce a Costal Cartilage Model for Simulation of Auricular Reconstruction. Otolaryngol Head Neck Surg 2016;155:356-9. [Crossref] [PubMed]
- Barber SR, Kozin ED, Dedmon M, et al. 3D-printed pediatric endoscopic ear surgery simulator for surgical training. Int J Pediatr Otorhinolaryngol 2016;90:113-8. [Crossref] [PubMed]
- Cote V, Schwartz M, Arbouin Vargas JF, et al. 3-Dimensional printed haptic simulation model to teach incomplete cleft palate surgery in an international setting. Int J Pediatr Otorhinolaryngol. 2018;113:292-7. [Crossref] [PubMed]
- Gadaleta DJ, Huang D, Rankin N, et al. 3D printed temporal bone as a tool for otologic surgery simulation. Am J Otolaryngol 2020;41:102273. [Crossref] [PubMed]
- Sparks D, Kavanagh KR, Vargas JA, et al. 3D printed myringotomy and tube simulation as an introduction to otolaryngology for medical students. Int J Pediatr Otorhinolaryngol 2020;128:109730. [Crossref] [PubMed]
- Rose AS, Kimbell JS, Webster CE, et al. Multi-material 3D Models for Temporal Bone Surgical Simulation. Ann Otol Rhinol Laryngol 2015;124:528-36. [Crossref] [PubMed]
- Ha JF, Morrison RJ, Green GE, et al. Computer-Aided Design and 3-Dimensional Printing for Costal Cartilage Simulation of Airway Graft Carving. Otolaryngol Head Neck Surg 2017;156:1044-7. [Crossref] [PubMed]
- Rooney DM, Tai BL, Sagher O, et al. Simulator and 2 tools: Validation of performance measures from a novel neurosurgery simulation model using the current Standards framework. Surgery 2016;160:571-9. [Crossref] [PubMed]
- Low CM, Morris JM, Price DL, et al. Three-Dimensional Printing: Current Use in Rhinology and Endoscopic Skull Base Surgery. Am J Rhinol Allergy 2019;33:770-81. [Crossref] [PubMed]
- Akhtar K, Sugand K, Sperrin M, et al. Training safer orthopedic surgeons. Construct validation of a virtual-reality simulator for hip fracture surgery. Acta Orthop 2015;86:616-21. [Crossref] [PubMed]
- Puram SV, Kozin ED, Sethi RK, et al. Influence of trainee participation on operative times for adult and pediatric cochlear implantation. Cochlear Implants Int 2015;16:175-9. [Crossref] [PubMed]
- Meier JC, Remenschneider AK, Gray ST, et al. The impact of surgical trainee participation on sinus surgery outcomes. Laryngoscope 2016;126:316-21. [Crossref] [PubMed]
- Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016;159:1485-500. [Crossref] [PubMed]
- Barber SR, Kozin ED, Naunheim MR, et al. 3D-printed tracheoesophageal puncture and prosthesis placement simulator. Am J Otolaryngol 2018;39:37-40. [Crossref] [PubMed]
- Schwartz MA, Kavanagh KR, Frampton SJ, et al. Using simulators to teach pediatric airway procedures in an international setting. Int J Pediatr Otorhinolaryngol 2018;104:178-81. [Crossref] [PubMed]
Cite this article as: Friedland Y, Cheah T, Green GE, Zopf DA, Ha JF. Computer-aided design and 3D printing for airway graft carving simulation and the effect on participants performance and confidence. Aust J Otolaryngol 2021;4:1.