Tympanic paragangliomas: a large case series and single institutional experience
Introduction
Tympanic paragangliomas (TPGs) are rare, histologically benign but locally invasive neoplasms originating in the middle ear (1-5). Until reports by Guild and Rosenwasser they were not widely recognised or understood (6-8). TPGs are a subset of head and neck paragangliomas (HNPGs), which are uncommon neuroendocrine tumors (9), and derived from neural crest cells associated with parasympathetic paraganglia (10). TPGs arise along the tympanic branch of the glossopharyngeal nerve or less commonly the auricular branch of the vagus nerve (3,5).
TPGs commonly present with pulsatile tinnitus and/or hearing loss (1-4). They characteristically demonstrate slow growth, which can be unpredictable, and eventually lead to complications (11-14). For TPGs, surgical treatment is still commonly held as the ideal management approach with the technical aspects being well established (3,4,15,16). However, there is still a lack of consensus about the optimal management of HNPGs (17). Our understanding of HNPGs, in particular the genetics of paragangliomas, has evolved rapidly over the last few decades (11,18). As a result, the approach to the assessment of TPGs has significantly changed in light of this (10). In spite of this, clinicopathologic and treatment data for TPGs is sparsely reported in the literature. Our study presents one of the largest longitudinal series of TPGs with a focus on the clinicopathologic factors and treatment outcomes.
We present this following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/ajo-20-71)
Methods
Study cohort
Guy’s and St Thomas’ Hospital Trust London, United Kingdom maintains a prospective clinicopathological database of HNPGs. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Quality Improvement and Patient Safety Committee at Guy’s and St Thomas’ Hospital Trust and individual consent for this retrospective analysis was waived. A retrospective review of the HNPG database was performed and this identified 349 cases with HNPGs managed from 1984 until 2019. Inclusion criteria for this study were all patients with a clinical or pathologic diagnosis of a TPG, giving 59 cases. Exclusion criteria included any patient with no demographic or clinicopathologic details in the database, and so 3 cases were excluded leaving a total of 56 cases suitable for analysis.
Clinicopathologic and treatment factors
A literature review was performed to determine significant clinical, pathologic, and treatment factors for TPGs including: gender, age at diagnosis, tumor location, multifocality, genetic testing, hormone secretion, malignancy, follow-up date, death date, and cause of death.
The database listed a small number of cases with tumors that could be regarded as facial paragangliomas, and these were included in the tympanic paraganglioma group.
Treatment factors included: treatment type, treatment date, and treatment failure. The initial treatment window was defined as 2 years from first treatment date for any intervention or from diagnosis if an intervention was planned. The reason for this is that if multimodality or staged treatment was planned then it can take time to complete. Treatment failure, or progression, was defined as recurrence requiring further intervention after 2 years from initial treatment, or metastasis or death at any time after initial treatment has occurred.
Statistical analysis
The database is compiled into Excel Version 16.6.11 (Microsoft Corp., Redmond, Washington, United States of America) for Mac (Cupertino, California, United States of America) and analysed using Stata 16.0 (Statacorp, College Station, Tx, USA).
Time from diagnosis to progression was calculated, and patients who did not progress were censored at the last date of follow-up when no progression was confirmed. The Kaplan-Meier method was used to calculate disease free-survival probabilities, and the logrank test was used to formally test whether predictor covariates of interest were associated with progression. Factors tested included age at presentation, sex, whether or not the patient had multiple lesions, whether the tumour was secreting or had mutations, tumour size, and treatment modality. Malignancy was not tested as there were no malignant TPGs.
An analysis of treatment failure correlated to clinical and treatment factors was performed. The analysis was of 54 cases of TPGs as two were censored due to incomplete data. No statistically significant clinicopathologic or treatment factors were found that correlated with treatment failure for TPGs.
Results
Clinicopathologic
This study included 56 patients with TPGs, and each patient had only one TPG. The average age at presentation was 53.4 years (SD ±13.1), with a male to female ratio of 1:17.7 (Table 1).

Full table
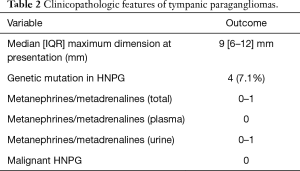
Clinicopathologic features are detailed in Table 2. There was a slight right sided predominance of tumors at 52.9% seen in our series. The median tumor maximum dimension at presentation was 9 mm with interquartile range (IQR) of 6–12 mm. There was a genetic mutation or variants of uncertain significance (VUS) associated with TPGs found in four (7.1%) patients. Five (8.9%) patients with TPGs had multiple paragangliomas. Of all patients with TPGs, three (5.4%) had other HNPGs and two (3.6%) had other non-HNPGs.
Full table
When patients have multiple paragangliomas there is uncertainty about which tumor is the source of the secretions, therefore it is only possible to give a range of the proportion of secretory tumors. A number of patients with TPGs, where secretion was detected, had multiple tumors including some with non-HNPGs. The proportion of tumors that were secretory for TPG ranged from 0–1.8%.
There were no TPGs associated with malignancy in this cohort of patients.
Hearing outcomes
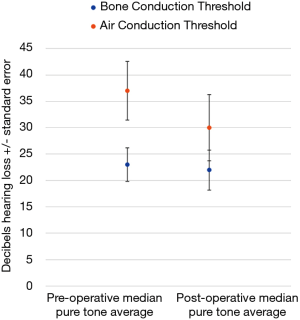
There were 21 patients who had pre- and post-operative audiograms, and this data is illustrated in Figure 1. Air conduction (AC) and bone conduction (BC) thresholds were assessed across frequencies from 250 Hz, 500 Hz, 1 kHz, 2 kHz, 4 to 8 kHz both pre- and post-operatively. A pure tone average (PTA) was calculated using those frequencies. The median pre-operative AC PTA was 37 (IQR, 14–50) Decibels hearing level (dBHL). The median pre-operative BC PTA was 23 (IQR, 12.5–31) dBHL. The median post-operative AC PTA was 30 (IQR, 18–67) dBHL. The median post-operative BC PTA was 22 (IQR, 13.5–38.5) dBHL. The difference between the medians pre- and post-operative AC and BC PTAs was a 7 Db and 1 decibels improvement respectively. Only one patient suffered a greater than 20Db worsening in hearing thresholds, with a 61 Decibel deterioration of their PTA post-operatively. No patients ended up with an anacoustic ear.
Treatment
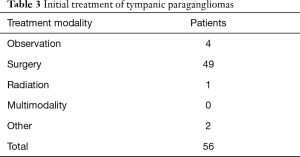
Details of treatment for the TPGs is outlined in Table 3. The mainstay of treatment was surgery, which was performed on 49 tumors (87.5%). Four cases were observed and the initial treatment for 2 cases could not be determined due to lack of data, and these were classified as ‘Other’ in Table 3. In one case treatment was by radiation and this was performed 40 years ago with the reasoning behind this treatment decision being unclear. The median tumor follow-up to progress or censoring was for 40 months (IQR, 15.8–89.3).
Full table
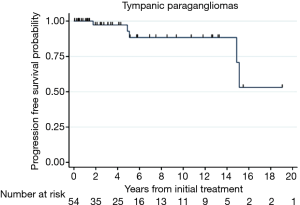
Progression free survival or treatment failure was calculated for TPG in Figure 2, with progression free survival probability at 2 years: 0.97 (95% CI: 0.82–0.996), at 5 years: 0.93 (95% CI: 0.73–0.98), and at 10 years: 0.88 (95% CI: 0.68–0.96). The incidence rate for failure for the entire sample is 5 over 288.2 PYFU; or 1.73 (95% CI: 0.872–4.2)/100 PYFU.
Complications
If hearing loss is excluded then complications included 4 tympanic membrane perforations, 1 case of external auditory canal stenosis and 1 case chronic mastoid cavity infection.
Discussion
The natural history of TPGs is usually one of indolent growth however, HNPGL growth can at times be unpredictable (10,12,19). Due to their inherent proximity to the facial nerve and inner ear, the onset of facial palsy and sensorineural hearing loss may be associated with tumors of a small size (4). The aim of surgical intervention is to remove the tumor and thus prevent the complications of progressive tumor enlargement and to alleviate symptoms. For these reasons, treatment of TPGs is by surgery where possible, and conservative management is generally offered to patients unfit for surgery (3,4). Surgery for TPGs usually is curative and has a low morbidity profile and complete resection minimises the risk of morbidity secondary to the tumor, such as hearing loss or facial nerve palsy (2,4,20).
Clinical factors
The literature reports two different statistics with regards to age: (I) age at presentation and (II) age at first treatment, however it is often unclear which is being reported. The average age of TPGs reported in the literature range from 52.6–60.3 years, and a bimodal distribution has been reported (2-4,14,21). This age range was consistent with the average age of presentation in our series of 53.4 years (SD ±13.1), and age was normally distributed in this study.
Our series noted a slight right sided predominance at 52.9%, but this has not been observed in the literature (2-4).
A female preponderance has been reported in the literature for HNPGLs, with the overall male to female ratio of ranging from 1:1.7–4 (14,17,21-23). The gender distribution for the different HNPG subtypes varies, with females more commonly developing TPGs followed by jugular paragangliomas, vagal paragangliomas and carotid body tumors. The male to female sex distribution for TPGs in the literature ranges from 1:2.7–9.5, while it is higher in our series at 1:17.1 (3,4,14,15,21). There is a low germline succinate dehydrogenase (SDH) mutation rate associated with TPGs (3,14). This may explain the female preponderance, as male gender has been reported as a predictor for a mutation in SDH gene (24).
The median maximum tumor dimension at presentation in our series was determined via radiological means as the maximum dimension in any plane either axial, sagittal or coronal. TPGs are often diagnosed while small and our series supports this finding. This is thought to be due to TPGs eliciting symptoms, such as hearing loss or pulsatile tinnitus, at an earlier stage compared to other HNPG.
In our series, 5 (8.9%) patients with TPGs had multiple paragangliomas and this comprised of both HNPGs and non-HNPGs. There were no bilateral TPGs observed in our patients. In the literature, rates of multiplicity in cases with TPGs is low (4). This is explained by the fact that SDH mutations increase the rates of multifocal tumors and there is a low mutation rate observed for TPGs (3,14).
Pathologic factors
Genetic testing for HNPGs is relatively recent occurrence, after the discovery of the SDH variant association identified in hereditary paragangliomas in 2000 (25). Genetic screening now is an important part of the assessment and management for most HNPGs (10,18). However, there is no consensus regarding genetic testing in HNPGs, with some clinicians recommending selective testing while others advocating universal testing for all HNPGs (11,18,24,26). At our institution genetic testing for HNPGL is being utilised with increasing frequency. Not all patients in this study underwent genetic testing because our series predates the introduction of genetic testing.
Pathogenic variants in SDH identified in patients with HNPG is associated with a positive family history, multiple tumors, younger age at presentation and male gender (24). These trends were not clearly seen in our series due to the low rates of pathologic SDH variants associated with TPG (7.1%). The lack of family history or pathogenic variants associated with TPG are consistent with the literature (3,14). Based on this study screening patients with TPGs for other paragangliomas or a predisposing germline mutation though not routinely needed should at least be considered on a case by case basis.
Metanephrines or metadrenalines can be tested in urine or plasma, with the latter test being easier and having a higher sensitivity and specificity (27). HNPGs are associated with the parasympathetic nervous system while non-HNPG are associated with the sympathetic nervous system and this accounts for the lower rates of secretion in HNPGs (28). The proportion of cases for all HNPGs where metanephrine secretion is detected is estimated in the literature to be 1–6% (14,17,21,29). Metanephrine secretion is almost never associated with isolated TPGs with the proportion secreting reported at less than 1%. The reason for this is uncertain but it may be related to the small size of TPGs (2-4,30). The proportion of metanephrine secreting TPGs in our series is estimated to range from 0–1.8% (Table 2). The range reflects the uncertainty around identifying the source of secretion in patients with multiple tumors. The lower limit of the range represents the proportion of secreting isolated TPGs. The upper limit of the range theoretically equals the proportion of secreting TPGs if in patients with multiple HNPGs then the TPG is always the source of metanephrines.
A limitation of our series is that it may underestimate secretion rates as there was incomplete testing of patients with TPGs and also some patients were tested using urinary tests which are less sensitive.
There were no TPGs associated with malignant change in our series (Table 2). Malignancy in paragangliomas can be difficult to determine as there are no definitive histopathologic criteria, but is defined by metastatic spread which in turn can be complicated by the multifocal nature of HNPGs (17,18). The overall malignancy rate in the literature for HNPGs varies widely from 0–19%, and differs by subtype (9,14,17,21,22,31,32). The rates of malignancy directly correlate with the proportion of genetic variants seen in each HNPG subtype (11,33,34), and that would help explain the fact that TPGs are not usually associated with malignancy (4).
Audiologic outcomes
TPGs commonly cause hearing loss. The hearing loss may be conductive or mixed hearing loss as TPGs often impinge on the ossicular chain and tympanic membrane (3). Less commonly there can be involvement of the inner ear causing a sensorineural hearing loss (4). In general, surgery would be expected to correct any conductive hearing loss and prevent a sensorineural hearing loss developing as a result of a TPG. Due to the location of TPGs there is a small but significant risk to hearing with surgery. In this study hearing thresholds were relatively well preserved, with the difference between pre- and post-operative median AC and BC mean PTA thresholds levels being a 7 Db and 1 Db improvement respectively, as seen in Figure 1. In the literature, reported hearing outcomes vary after surgery. Overall, with respect to hearing levels after surgery for TPGs there may be a small improvement, or deterioration, or they may remain unchanged, with a poor hearing outcome being an infrequent complication (3,4,15).
Treatment
The majority of patients (87.5%) in our series were treated with surgery with only 5 treatment failures, and this is similar to other series in the literature (3,4). The TPGs in our series were, on the whole, primarily treated at our institution. Of the 5 failures 40% (n=2) were both treated and then successfully salvaged with surgery at our institution. Another 40% (n=2) were operated on elsewhere and referred due to a recurrence requiring surgery. The last failure was a patient with a TPG who initially requested conservative management, but then after 15 years of observation required surgery due to progressive tumor growth and associated symptoms. Exclusion of those last 2 categories of failures, either initially treated externally or who refused surgical management, would produce an even lower failure rate. Thus, surgical management of TPGs at a high-volume centre leads to excellent outcomes. There is a low recurrence rate and good long-term control reported in the literature which is comparable to that in this study (2-4,35). The low rate of treatment failure or disease progression limits a statistical analysis of factors contributing to failure.
The key to surgical management is a thorough pre-operative clinical and radiological assessment (3). The vascular supply to the TPGs is usually the inferior tympanic branch of the ascending pharyngeal artery, but may receive supply from other arteries including the caroticotympanic branches of the internal carotid artery (15). The surgical approach for TPGs is commonly via either a transcanal or transmastoid approach (2-4,10,14,16,36), with an emphasis on surgical access and addressing the tumor vascular supply (15,16).
Based on the outcomes reported in this study and the literature regarding TPGs, a minimum 5-year period of clinical follow-up after treatment would be advised (2,4,37,38). Post-operative imaging would be recommended if the patient becomes symptomatic or is suspected of developing another HNPGL. Surveillance image may also be required in patients who are at risk of developing other paragangliomas (10,39-41).
Complications
Intra-operative complications are uncommonly reported in surgery for TPGs (3,4), however rare and serious complications have been reported (4). If changes in hearing level are excluded, our series had a low rate of complications at 10.7% with most being relatively minor in nature, such as tympanic membrane perforation or post-operative wound infection. As discussed previously only one patient had a greater than 20 Db deteriorating in hearing thresholds.
Our series has a number of limitations including being retrospective in nature and lacking a complete data set. Also approach to management of TPGs has evolved over time and this inherently reflected in our series. Despite these limitations our series provides insights into clinicopathologic and treatment of a rare disease.
Conclusions
TPGs are rare middle ear tumors that require a careful and thorough assessment. This study shows TPGs are associated with other paragangliomas and also germline SDH mutations, and that surgical treatment is safe and effective strategy and should be used where possible. Improved understanding of these tumors will hopefully lead to better patient outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-20-71
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ajo-20-71
Peer Review File: Available at http://dx.doi.org/10.21037/ajo-20-71
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-20-71). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Quality Improvement and Patient Safety Committee at Guy’s and St Thomas’ Hospital Trust and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neto ME, Vuono IM, Souza LR, et al. Tympanic paragangliomas: case reports. Braz J Otorhinolaryngol 2005;71:97-100. [Crossref] [PubMed]
- Misale P, Lepcha A, Tyagi AK. Glomus Tympanicum: Clinical Presentation, Management and Outcomes. Indian J Otol 2018;24:56-9. [Crossref]
- O'Leary MJ, Shelton C, Giddings NA, et al. Glomus tympanicum tumors: a clinical perspective. Laryngoscope 1991;101:1038-43. [PubMed]
- Carlson ML, Sweeney AD, Pelosi S, et al. Glomus tympanicum: a review of 115 cases over 4 decades. Otolaryngol Head Neck Surg 2015;152:136-42. [Crossref] [PubMed]
- Weissman JL, Hirsch BE. Beyond the promontory: the multifocal origin of glomus tympanicum tumors. AJNR Am J Neuroradiol 1998;19:119-22. [PubMed]
- Rosenwasser H. Carotid body tumor of the middle ear and mastoid. Arch Otolaryngol 1945;64-7. [Crossref]
- Guild SR. A hitherto unrecognized structure, the glomus jugularis, in man. Anat Rec 1941;1941:28.
- Ruben RJ. The history of the glomus tumors-nonchromaffim chemodectoma: a glimpse of biomedical Camelot. Acta Otolaryngol 2007;127:411-6. [Crossref] [PubMed]
- Anttila T, Hayry V, Nicoli T, et al. A two-decade experience of head and neck paragangliomas in a whole population-based single centre cohort. Eur Arch Otorhinolaryngol 2015;272:2045-53. [Crossref] [PubMed]
- Taïeb D, Kaliski A, Boedeker CC, et al. Current approaches and recent developments in the management of head and neck paragangliomas. Endocr Rev 2014;35:795-819. [Crossref] [PubMed]
- Baysal BE, Maher ER. 15 years of paraganglioma: genetics and mechanism of pheochromocytoma-paraganglioma syndromes characterized by germline SDHB and SDHD mutations. Endocr Relat Cancer 2015;22:T71-82. [Crossref] [PubMed]
- Jansen JC, van den Berg R, Kuiper A, et al. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer 2000;88:2811-6. [Crossref] [PubMed]
- Suárez C, Rodrigo JP, Bodeker CC, et al. Jugular and vagal paragangliomas: Systematic study of management with surgery and radiotherapy. Head Neck 2013;35:1195-204. [Crossref] [PubMed]
- Hayward NC, Head N. Hearing and Balance Medicine 2017;1:1-12.
- House WF, Glasscock ME 3rd. Glomus tympanicum tumors. Arch Otolaryngol 1968;87:550-4. [Crossref] [PubMed]
- Jethanamest D. Tympanic paraganglioma—Resection techniques. Operative Techniques in Otolaryngology 2016;27:15-9. [Crossref]
- Papaspyrou K, Mewes T, Rossmann H, et al. Head and neck paragangliomas: Report of 175 patients (1989-2010). Head Neck 2012;34:632-7. [Crossref] [PubMed]
- Boedeker CC, Hensen EF, Neumann HP, et al. Genetics of hereditary head and neck paragangliomas. Head Neck 2014;36:907-16. [Crossref] [PubMed]
- Carlson ML, Sweeney AD, Wanna GB, et al. Natural history of glomus jugulare: a review of 16 tumors managed with primary observation. Otolaryngol Head Neck Surg 2015;152:98-105. [Crossref] [PubMed]
- Cosetti M, Linstrom C, Alexiades G, et al. Glomus tumors in patients of advanced age: a conservative approach. Laryngoscope 2008;118:270-4. [Crossref] [PubMed]
- Smith JD, Harvey RN, Darr OA, et al. Head and neck paragangliomas: A two-decade institutional experience and algorithm for management. Laryngoscope Investig Otolaryngol 2017;2:380-9. [Crossref] [PubMed]
- González-Orús Álvarez-Morujo RJ, Aristegui Ruiz MA, da Costa Belisario J, et al. Head and neck paragangliomas: Experience in 126 patients with 162 tumours. Acta Otorrinolaringol Esp 2015;66:332-41. [PubMed]
- Pindicura KA, R, Dandala S, Rajarikam N, Vivekanand N. Paragangliomas of the head and neck region: A single center experience. Journal of Dr NTR University of Health Sciences 2017;6:19-23.
- Neumann HP, Erlic Z, Boedeker CC, et al. Clinical predictors for germline mutations in head and neck paraganglioma patients: cost reduction strategy in genetic diagnostic process as fall-out. Cancer Res 2009;69:3650-6. [Crossref] [PubMed]
- Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000;287:848-51. [Crossref] [PubMed]
- Hussain I, Husain Q, Baredes S, et al. Molecular genetics of paragangliomas of the skull base and head and neck region: implications for medical and surgical management. J Neurosurg 2014;120:321-30. [Crossref] [PubMed]
- Hickman PE, Leong M, Chang J, et al. Plasma free metanephrines are superior to urine and plasma catecholamines and urine catecholamine metabolites for the investigation of phaeochromocytoma. Pathology 2009;41:173-7. [Crossref] [PubMed]
- Baysal BE. Hereditary paraganglioma targets diverse paraganglia. J Med Genet 2002;39:617-22. [Crossref] [PubMed]
- Erickson D, Kudva YC, Ebersold MJ, et al. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab 2001;86:5210-6. [Crossref] [PubMed]
- Kouzaki H, Fukui J, Shimizu T. Management of a catecholamine-secreting tympanicum glomus tumour: case report. J Laryngol Otol 2008;122:1377-80. [Crossref] [PubMed]
- Moskovic DJ, Smolarz JR, Stanley D, et al. Malignant head and neck paragangliomas: is there an optimal treatment strategy? Head Neck Oncol 2010;2:23. [Crossref] [PubMed]
- Sethi RV, Sethi RK, Herr MW, et al. Malignant head and neck paragangliomas: treatment efficacy and prognostic indicators. Am J Otolaryngol 2013;34:431-8. [Crossref] [PubMed]
- Burnichon N, Rohmer V, Amar L, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Clin Endocrinol Metab 2009;94:2817-27. [Crossref] [PubMed]
- Rinaldo A, Myssiorek D, Devaney KO, et al. Which paragangliomas of the head and neck have a higher rate of malignancy? Oral Oncol 2004;40:458-60. [Crossref] [PubMed]
- Forest JA 3rd, Jackson CG, McGrew BM. Long-term control of surgically treated glomus tympanicum tumors. Otol Neurotol 2001;22:232-6. [Crossref] [PubMed]
- Hu K, Persky MS. Treatment of Head and Neck Paragangliomas. Cancer Control 2016;23:228-41. [Crossref] [PubMed]
- Singh VK, Badhwar S, D'Souza J, et al. Glomus Tympanicum. Med J Armed Forces India 2004;60:200-3. [Crossref] [PubMed]
- Papaspyrou K, Mann WJ, Amedee RG. Management of head and neck paragangliomas: review of 120 patients. Head Neck 2009;31:381-7. [Crossref] [PubMed]
- Eijkelenkamp K, Osinga TE, de Jong MM, et al. Calculating the optimal surveillance for head and neck paraganglioma in SDHB-mutation carriers. Fam Cancer 2017;16:123-30. [Crossref] [PubMed]
- Corssmit EP, Romijn JA. Clinical management of paragangliomas. Eur J Endocrinol 2014;171:R231-43. [Crossref] [PubMed]
- Capatina C, Ntali G, Karavitaki N, et al. The management of head-and-neck paragangliomas. Endocr Relat Cancer 2013;20:R291-305. [Crossref] [PubMed]
Cite this article as: Mclean T, Rudd J, Kerr SJ, Gleeson M, Obholzer R. Tympanic paragangliomas: a large case series and single institutional experience. Aust J Otolaryngol 2021;4:8.