Prognostic biomarkers of human papilloma virus (HPV)-positive neoplasia of the upper aerodigestive tract: a systematic review
Introduction
Head and neck malignancy is the sixth most common cancer globally with an incidence of half a million cases per year (1). More than 90% of these are squamous cell carcinoma (SCC), a genetically heterogenous neoplasm (2). Head and neck SCC is primarily associated with alcohol and tobacco consumption however human papilloma virus (HPV) infection is becoming increasingly common as a predisposing factor. HPV is thought to promote malignant transformation by surpassing cell cycle checkpoints and thus causing genomic instability. Inverted papilloma (IP) is a locally aggressive, benign sinonasal neoplasm, notable for its tendency for local recurrence and potential for malignant transformation. The incidence of malignant transformation varies in the literature but is largely regarded to be between 5% and 15% (3). Whilst HPV has been implicated in the pathogenesis of IP and its malignant transformation to SCC, studies have not consistently demonstrated a true connection between the virus and IP (4-6). The relationship between HPV and recurrence or malignant transformation of benign IP remains controversial.
In oropharyngeal SCC, HPV positivity represents a distinct biological entity, both in terms of its underlying genetics and clinical behaviour. Next generation sequencing has enabled researchers to begin identifying biomarkers associated with HPV-positive and negative SCC, which can aid in predicting tumour behaviour as well as offering potential targets for treatment in the future. HPV positive tumours have already been demonstrated to have lower mutation rates than their HPV-negative counterparts and mutated genes rarely overlap between these two groups (7,8).
P16, a cyclin dependent kinase inhibitor, is frequently underexpressed in HPV-negative oropharyngeal SCC and overexpressed in HPV positive tumours. Thus, P16 is utilized as a first-line test for determination of HPV status as well as serves as an independent prognostic tool (9,10).
Currently, the gold standard of care of HPV positive cancers involves either surgery with adjuvant radiotherapy +/− chemotherapy or definitive concurrent chemoradiotherapy (11,12). HPV positive oropharyngeal SCC is associated with an improved prognosis and better response to treatment. This finding has been replicated in sinonasal malignancy (13) but remains to be proven in other subsites. Despite this association, a small subset of HPV-positive patients have been demonstrated to have less favourable outcomes. Whilst tobacco smoking has been implicated as a risk factor for poorer outcomes (14,15), few other poor prognostic factors in HPV-positive SCC have been described in the literature. Approximately 10% of HPV positive patients are at high risk of developing distant metastasis and whilst this rate is similar to that of HPV-negative tumours, metastasis in HPV positive cancers tend to occur later and be more disseminated (16-18). This study aims to systematically review the literature and assess the evidence for known biomarkers in HPV positive neoplasia of the upper aerodigestive tract (UADT), that may aid in predicting which patients may go on to have poorer outcomes or respond poorly to treatment.
Methods
A systematic review was performed to evaluate the literature regarding prognostic biomarkers in HPV positive neoplasia of the UADT. The methods of this review were in keeping with PRISMA guidelines (19) and/or the Cochrane Handbook for Systematic Reviews of Interventions where applicable (20).
Eligibility criteria
Studies containing original data pertaining prognostic outcomes of any biomarkers in HPV positive neoplasia of the UADT were considered for inclusion in this study. HPV-positive status was defined by polymerase chain reaction (PCR), in situ hybridization (ISH) or P16 immunohistochemistry (IHC). No age or comorbidity restrictions were applied. Studies assessing biomarkers in both HPV-positive and negative neoplasia were included only if they reported extractable data pertaining specifically to HPV-positive tumours. Case series, case-control studies, crossover studies, cohort studies and randomized controlled trials (RCTs) were included. Only manuscripts published in English were eligible; reviews, guidelines, letters, and editorials with no original data were excluded, as were case reports, conference abstracts, in vitro and animal studies.
Information sources
A systematic electronic search was performed until December 19th 2015 on the Embase (1974–2015), Medline (1946–2015) databases as well as Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, current issue), Cochrane Ear, Nose and Throat Disorders Group Trials Register and mRCT (metaRegister of Controlled Trials including www.ClinicalTrials.gov). Reference lists of identified publications were scanned for additional studies.
Search methods
A search strategy was designed for each database (Table 1) to identify all studies evaluating prognostic biomarkers of HPV-positive neoplasia of the UADT.
Table 1
| Papilloma |
| [1] exp Papilloma, inverted |
| [2] exp Papilloma |
| [3] (epithelial papilloma OR schneiderian papilloma OR papillary sinusitis OR soft papilloma OR transitional cell papilloma OR inverting papilloma OR inverted papilloma).mp |
| [4] [1] OR [2] OR [3] |
| SCC |
| [5] exp Carcinoma, squamous cell |
| [6] (dysplasia OR metaplasia OR atypia OR carcinoma in situ) |
| [7] (cancer* or carcinoma* or neoplas* or tumor* or tumour* or malignan* or SCC*).mp |
| [8] [5] OR [6] OR [7] |
| Nasal cavity |
| [9] exp Nose |
| [10] exp Nasal cavity |
| [11] exp nasal mucosa |
| [12] exp paranasal sinuses |
| [13] exp paranasal sinus diseases |
| [14] exp nasopharynx |
| [15] (nose OR nasal$ OR sinus$ OR rhinosinus$ OR paranasal$ OR rhiniti$ OR nasosinus$ OR pansinus$).mp |
| [16] [9] OR [10] OR [11] OR [12] OR [13] OR [14] OR [15] |
| Oral cavity and oropharynx |
| [17] exp Mouth |
| [18] exp Lip |
| [19] exp Tongue |
| [20] exp Mouth mucosa |
| [21] exp Salivary glands |
| [22] (oral$ or intra-oral$ or intraoral$ or “intra oral$” or gingiva$ or oropharyn$ or mouth$ or tongue$ or cheek$ or gum$ or palatal$ or palate$ or “head and neck”).mp. |
| [23] [17] OR [18] OR [19] OR [20] OR [21] OR [22] |
| HPV |
| [24] exp tumor virus |
| [25] exp papillomavirus infections |
| [26] (hpv* or papillomavir* or (papilloma* and vir*) OR human papilloma virus).mp. |
| [27] [24] OR [25] OR [26] |
| [43] [4] OR [8] |
| [44] [16] OR [23] |
| [37] [43] AND [44] AND [27] |
SCC, squamous cell carcinoma; HPV, human papilloma virus.
Study selection
One author (PL Sacks) reviewed and selected trials found in the searches and evaluated them against the inclusion criteria. In cases where PL Sacks was unsure as to whether the trial was relevant, a second review author (R Harvey) was consulted. Initial screening was upon title review, with brief abstract review if there was uncertainty. The remaining selection underwent stringent abstract review, with discussion between reviewers if uncertain about relevance of individual studies. The full texts of the subsequent selection were analyzed, with study exclusion if not relevant.
Data extraction
A structured data collection form was used for data extraction. The data extraction sheet was pilot-tested on ten randomly-selected included studies and refined accordingly. One review author (PL Sacks) extracted the following data from included studies and a second author (R Harvey) was consulted if any uncertainty arose:
- Study characteristics including study design, inclusion and exclusion criteria, total number of patients, total number of HPV-positive patients, HPV detection method, primary intervention, outcomes assessed, biomarkers assessed and length of follow-up;
- Population demographics including age, gender, smoking and alcohol status, diagnosis, stage at diagnosis;
- Outcomes including percentage of biomarker expressed in population, overall survival, disease-specific survival, recurrence, response to treatment and distant metastasis.
Summary measures
Proportions of individual biomarker expression were calculated manually. Hazard ratios and 95% confidence intervals for each prognostic outcome were extracted from manuscripts when available.
A qualitative synthesis was performed where a thematic organisation was created based on endpoint of each study. Outcomes assessed included treatment effects, severity effects and overall mortality.
Results
Search results
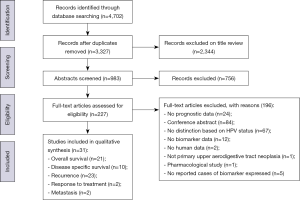
The search strategy found 4,702 records. This was reduced to 3,327 after the removal of duplicates. Title and abstract review excluded a further 3,100 articles, leaving 227 full text articles to be assessed for eligibility. There were 31 studies included in the final qualitative synthesis (Figure 1). All 31 articles were case series and all investigated malignant disease of the UADT. Twenty-four articles looked exclusively at oropharyngeal or oral SCC. Characteristics of included studies are described in Table 2.
Table 2
| Author | Year | Study design | Diagnosis | Primary intervention | Total no. patients (n) | No. HPV+ patients (n) | HPV diagnosis method | Age (mean/median) (years) | Gender (% female) | % smokers | Biomarker(s) assessed |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qian et al. (21) | 2015 | Case series | OPSCC | NR | 96 | 68 | NR | 57 | 17.7% | 80.2% | Heregulin, HER3 |
| Zhang et al. (22) | 2015 | Case series | OPSCC | NR | 1,008 | 233 | PCR or ISH | 55.8 | 13.5% | 75.5% | SNP in promoter region of FAS and FASLG |
| Balermpas et al. (23) | 2014 | Case series | All HNSCC | Radiotherapy | 106 | 42 | P16 IHC | 60.6 | 20.7% | 55% | CD68+, CD163+, CD11B+ |
| Kim et al. (24) | 2014 | Case series | OPSCC | Chemotherapy | 74 | 21 | PCR | 70 | 7% | NR | P53, beta-tubulin, BCL2, ERCC1 |
| Ko et al. (25) | 2014 | Case series | Oral and OPSCC | Surgery | 167 | 36 | ISH | 56 | 18.6% | 65.5% | miR21 |
| Liu et al. (26) | 2014 | Case series | OPSCC | Surgery and Radiotherapy | 105 | 48 | PCR/P16 IHC | 58.5 | 20% | 75.2% | Ki67 |
| Ryu et al. (27) | 2014 | Case series | Tonsillar SCC | Surgery | 42 | 30 | PCR | 58 | 9.5% | 64.3% | Cyclin D1, pRB, p53 |
| Tertipis et al. (28) | 2014 | Case series | Tongue SCC | Radiotherapy | 278 | 207 | Multiplex assay | 60 | 24.6% | 64.7% | LMP10 |
| Vainshtein et al. (29) | 2014 | Case series | Stage III or IV OPSCC | Radiotherapy | 198 | 184 | PCR or ISH | 55 | 10.9% | 56.6% | EGFR |
| Zhang et al. (30) | 2014 | Case series | OPSCC | NR | 846 | 158 | PCR or ISH | 55.6 | 13.1% | 62.7% | TNF-alpha |
| Bauman et al. (31) | 2013 | Case series | Stage III–IV HNSCC | Chemo-radiotherapy | 90 | 56 | P16 IHC | NR | 12% | 77% | ERCC1 |
| Chandarana et al. (32) | 2013 | Case series | OP and oral SCC | Radiotherapy | 85 | 26 | P16 IHC | 57.2 (oral), 52.3 (OP) | 74.1% | 88.2% | EGFR |
| Chiosea et al. (33) | 2013 | Case series | HPV+ OPSCC | Chemo-radiotherapy | 75 | 75 | ISH | 56 | 14.7% | 53.3% | PIK3CA |
| Kaka et al. (34) | 2013 | Case series | HPV+ OPSCC | Chemo-radiotherapy | 15 | 15 | P16 IHC/CISH | 59 | 14% | 57% | P53, NOTCH |
| Scantlebury et al. (35) | 2013 | Case series | OPSCC | Surgery | 202 | 150 | RNA ISH/P16 IHC | 56.8 | 11.9% | 70.8% | Cyclin D1 |
| Song et al. (36) | 2013 | Case series | OPSCC | NR | 658 | 102 | PCR | 55.3 | 14.4% | 63.4% | SNP in nucleotide excision repair pathway |
| Badoual et al. (37) | 2012 | Case series | All HN SCC | NR | 64 | 32 | INNO-LiPA genotyping extra assay |
NR | 34% | NR | PD-1-positive cells, CD8+, CD4+ |
| Gubanova et al. (38) | 2012 | Case series | OPSCC | NR | 40 | 20 | PCR | 58.2 | 20% | 47.5% | SMG1, ATM, ATR |
| Husain et al. (39) | 2012 | Case series | All HNSCC | NR | 101 | 29 | P16 IHC | NR | 18.4% | 80.7% | EGFR |
| Lim et al. (40) | 2012 | Case series | All HNSCC | Radiotherapy | 87 | 28 | P16 IHC | 56.6 | 13.7% | NR | ATM |
| Park et al. (41) | 2012 | Case series | OPSCC | Surgery | 86 | 46 | PCR | 62.1 | 16.3% | 69.8% | pRB, cyclin D1, CDK4, p21 |
| Hao et al. (42) | 2011 | Case series | SCC neck node unknown primary | Chemo-radiotherapy | 55 | 30 | CISH and PCR | 54.8 | 16.4% | 76.4% | ERCC1 |
| Moeller et al. (43) | 2011 | Case series | All HNSCC | Radiotherapy | 89 | 36 | PCR | 60 | 18% | 64% | Ku80 |
| Al-Swiahb et al. (9) | 2010 | Case series | OPSCC | Surgery | 220 | 33 | PCR | 51.3 | 42% | 79% | P53, EGFR |
| Hong et al. (44) | 2010 | Case series | OPSCC | NR | 270 | 99 | PCR/P16 IHC | 59.8 | 21% | NR | EGFR |
| Nichols et al. (45) | 2010 | Case series | OPSCC | Chemo-radiotherapy | 68 | 53 | ISH | NR | 14.7% | 51.5% | Bcl2 |
| Chung et al. (46) | 2009 | Case series | Stage IV tonsillar SCC | Chemo-radiotherapy | 46 | 23 | PCR | 53 | 13% | <50% | P53, pRB, p21 |
| Fallai et al. (47) | 2009 | Case series | OPSCC | Chemo-radiotherapy | 78 | 9 | PCR | 56.4 | 92% | NR | P53 |
| Fei et al. (48) | 2009 | Case series | Tonsillar SCC | NR | 85 | 42 | PCR or P16 IHC | 59 | 18.8% | NR | VEGF, EGFR |
| Klussmann et al. (49) | 2009 | Case series | OPSCC | Radiotherapy | 60 | 28 | PCR/P16 IHC | 60 | 22% | 80% | 11q13 amplification, 16q loss, 9p loss |
| Kumar et al. (50) | 2008 | Case series | Stage III and IV OPSCC | Chemotherapy | 42 | 25 | PCR | 62 | 24% | 78% | EGFR, P53, BCL-xL |
OPSCC, oropharyngeal squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; NR, not reported; HPV, human papilloma virus; PCR, polymerase chain reaction; ISH, in situ hybridization; IHC, immunohistochemistry; P16, protein 16 (inhibitor of cyclin dependent kinases); CISH, chromogenic in situ hybridization; RNA, ribonucleic acid; INNO-LiPA, innogenetics; HER3, human epidermal growth factor receptor 3; SNP, single nucleotide polymorphism; FAS, Fas cell surface death receptor; FASLG, FAS ligand; CD, cluster of differentiation; P53, protein 53; BCL2, B-cell lymphoma 2; ERCC1, excision repair cross-complementation group 1; miR21, microRNA-21; Ki67, marker of proliferation Ki-67; pRB, retinoblastoma protein; LMP10, low molecular weight protein 10; EGFR, epidermal growth factor receptor; TNF-alpha, tumour necrosis factor alpha; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; PD-1, programmed cell death protein 1; SMG1, suppressor with morphogenetic effect on genitalia; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; p21, protein 21; CDK4, cyclin dependent kinase 4; VEGF, vascular endothelial growth factor; BCL-xL, B-cell lymphoma-extra large.
Qualitative synthesis and thematic organisation
Study outcomes were organised into broad thematic groups. These included 21 studies evaluating overall survival, ten studies evaluating disease specific survival, 23 studies evaluating recurrence, two studies evaluating response to treatment and two studies evaluating risk of metastasis with some studies evaluating more than one outcome
Overall survival
There were 21 studies evaluating overall survival with a total number of 25 biomarkers investigated. There were five studies evaluating epidermal growth factor receptor (EGFR) with a total of 254 patients. Two studies (9,50) found that overexpression of EGFR was associated with worse overall survival whilst three studies (39,44,51) demonstrated no correlation amongst HPV-positive patients. There were four studies evaluating p53 with a total of 107 patients. Amongst HPV-positive patients, two studies (9,24) found that overexpression of p53 correlated with worse overall survival whilst two studies (27,46) found no correlation. Two studies (43,48) evaluated VEGF with a total of 78 patients and both found overexpression of VEGF to be associated with worse overall survival. Two studies (27,46) evaluated pRb with a total of 53 patients and both found no correlation with overall survival. Correlations of other biomarkers evaluated in less than two studies can be found in Table 3.
Table 3
| Biomarker | Studies (n) | Patients (n) | % of tumours expressing biomarker | Summary |
|---|---|---|---|---|
| EGFR | 5 | 254 | 50% | 2 studies—low expression EGFR associated with high OS: P=0.01, no HR reported (Al-Swiahb et al., 2010); P=0.03, no HR reported (Kumar et al., 2008) |
| 3 studies—no correlation with overall survival: P=0.4, no HR reported (Husain et al., 2012); P=0.22, HR 1.86 (95% CI, 0.68–5.13) (Qian et al., 2015); P=0.29, HR 1.42 (95% CI, 0.39–5.19) (Hong et al., 2010) | ||||
| p53 | 4 | 107 | 14% | 2 studies—low expression p53 associated with high OS: P≤0.01, no HR reported (Al-Swiahb et al., 2010); P=0.01, no HR reported (Kim et al., 2014) |
| 2 studies—no correlation with overall survival: P=0.48, no HR reported (Chung et al., 2009); P=0.43, HR 0.43 (95% CI 0.13-1.45) (Ryu et al., 2014) | ||||
| VEGF | 2 | 78 | 59.50% | 2 studies—high VEGF correlated with worse OS: P=0.06, HR 2.94 (95% CI, 0.94–12.91) (Fei et al., 2009); P=0.04, no HR reported (Moeller et al., 2011) |
| pRB | 2 | 53 | 11.30% | 2 studies—no correlation with OS: P=0.21, no HR reported (Chung et al., 2009); P=0.272, HR 0.47 (95% CI, 0.12–1.80)] (Ryu et al., 2014) |
| p21 | 1 | 23 | 78% | 1 study—no correlation with OS: P=0.66, no HR reported (Chung et al., 2009) |
| Cyclin D1 | 1 | 150 | 3.70% | 1 study—intensity of expression associated with better OS: P=0.038, no HR reported (Scantlebury et al., 2013) |
| ERCC1 | 1 | 30 | 50% | 1 study—no correlation with OS: P=0.58, HR 1.5 (95% CI, 0.3–6.8) (Hao et al., 2012) |
| PD-1+ve cells | 1 | 32 | 59% | 1 study—high numbers of PD-1+ T cells correlated with better OS: P=0.025, HR 0.13 (95% CI, 0.02–0.067) (Badoual et al., 2013) |
| CD8+ | 1 | 32 | 53% | 1 study—no correlation with better OS: P=0.6, HR 0.7 (95% CI, 0.14–3.6) (Badoual et al., 2013) |
| CD4+ | 1 | 32 | 68.70% | 1 study—no correlation with OS: P=0.7, HR 1.36 (95% CI, 0.22–8.6) (Badoual et al., 2013) |
| CD163+ | 1 | 42 | 42.90% | 1 study—no correlation with OS: P=0.112, no HR reported (Balermpas et al., 2014) |
| CD11B+ | 1 | 42 | 53.60% | 1 study—no correlation with OS: P=0.394, no HR reported (Balermpas et al., 2014) |
| SMG1 | 1 | 20 | 15% | High SMG1 expression correlated with poor OS: no P value nor HR (Gubanova et al., 2012) |
| Beta tubulin | 1 | 21 | 4.70% | Class III beta tubulin correlated with better OS: P=0.012, no HR reported (Kim et al., 2014) |
| 11q13 amp | 1 | 28 | 7.10% | 1 study—11q13 amp associated with worse OS: P=0.02, no HR reported (Klussman et al., 2009) |
| 16q loss | 1 | 28 | 28.60% | 1 study—16q loss associated with improved OS: P=0.01, no HR reported (Klussman et al., 2009) |
| 9p loss | 1 | 28 | 10.70% | 1 study—9p loss associated with worse OS: P<0.0015, no HR reported (Klussman et al., 2009) |
| Ku80 | 1 | 36 | NR | 1 study—no correlation with OS (data not reported) (Moeller et al., 2011) |
| CDK4 | 1 | 46 | 43.50% | 1 study—high CDK4 associated with worse OS: P=0.011, HR 2.91 (95% CI, 2.25–3.38) (Park et al., 2012) |
| Heregulin | 1 | 57 | 47.40% | 1 study—high heregulin correlated with worse OS: P=0.049, HR 3.30 (95% CI, 0.94–11.57) (Qian et al., 2015) |
| HER3 | 1 | 67 | 49.30% | 1 study—no correlation with OS: P=0.94, HR 0.82 (95% CI, 0.31–2.22) (Qian et al., 2015) |
| HER2 | 1 | 68 | 50% | 1 study—no correlation with OS: P=0.85, HR 1.10 (95% CI, 0.41–2.91) (Qian et al., 2015) |
| LMP10 | 1 | 207 | 44.44% nuclear | 1 study—nuclear [P=0.162, HR 1.673 (95% CI, 0.813–3.445)] nor cytoplasmic [P=0.164, HR 0.590 (95% CI, 0.281–1.240)] expression not correlated with OS (Tertipis et al., 2014) |
| 47.83% cytoplasm | ||||
| Bcl2 | 1 | 53 | 39.60% | 1 study—high Bcl2 correlated with worse OS: P=0.0064, HR 6.9 (95% CI, 1.7–27) (Nichols et al., 2010) |
| Ki67 | 1 | 48 | 54.10% | 1 study—no correlation with OS: P=0.144, HR 0.21 (95% CI, 0.08–0.56) (Liu et al., 2014) |
EGFR, epidermal growth factor receptor; p53, protein 53; VEGF, vascular endothelial growth factor; pRB, retinoblastoma protein; p21, protein 21; ERCC1, excision repair cross-complementation group 1; PD-1, programmed cell death protein 1; CD, cluster of differentiation; SMG1, suppressor with morphogenetic effect on genitalia; CDK4, cyclin dependent kinase 4; HER3, human epidermal growth factor receptor 3; LMP10, low molecular weight protein 10; BCL2, B-cell lymphoma 2; Ki67, marker of proliferation Ki-67; HR, hazard ratio; CI, confidence interval.
Disease specific survival
There were ten studies evaluating disease specific survival with a total number of eight biomarkers investigated. EGFR was evaluated in three studies, including a total of 93 patients and correlated with worse disease specific survival in all three studies (32,48,50). Correlations of other biomarkers evaluated in less than two studies can be found in Table 4.
Table 4
| Biomarker | Studies (n) | Patients (n) | % expressed | Summary |
|---|---|---|---|---|
| EGFR | 3 | 93 | 50.5% | 3 studies—EGFR correlated with worse DSS: P=0.04, no HR reported (Kumar et al., 2008); P=0.04, no HR reported (Fei et al., 2009); P=0.01, no HR reported (Chandarana et al., 2013) |
| p53 | 1 | 35 | 5% | 1 study—no correlation with DSS: P=0.272, no HR reported (Kim et al., 2014) |
| ERCC1 | 1 | 30 | 50% | 1 study—no correlation with DSS: P=0.85, HR 1.2 (95% CI, 0.2–8.5) (Hao et al., 2011) |
| MiR21 | 1 | 36 | 28% | 1 study—no correlation with DSS: P=0.486, no HR reported (Ko et al.,2014) |
| PIK3CA mutation | 1 | 75 | 31% | 1 study—no correlation with DSS: P=0.8, no HR reported (Chiosea et al., 2013) |
| CDK4 | 1 | 46 | 43% | 1 study—high CDK4 associated with worse DSM: P=0.007, HR 2.91 (95% CI, 2.25–3.38) (Park et al., 2012) |
| Cyclin D1 | 1 | 150 | 58.8% | 1 study—intensity of expression associated with worse DSS: P=0.015, no HR reported (Scantlebury et al., 2013) |
| Ki67 | 1 | 48 | 56% | 1 study—no correlation with OS: P=0.137, HR 0.23 (95% CI, 0.08–0.68) (Liu et al., 2014) |
EGFR, epidermal growth factor receptor; p53, protein 53; ERCC1, excision repair cross-complementation group 1; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; CDK4, cyclin dependent kinase 4; Ki67, marker of proliferation Ki-67; HR, hazard ratio; CI, confidence interval.
Locoregional recurrence
There were 23 studies evaluating rates of locoregional recurrence with 37 biomarkers investigated. There were four studies evaluating EGFR with a total of 354 patients. One study (48) found that overexpression of EGFR was associated with increased rates of recurrence whilst three studies (29,39,44) demonstrated no correlation amongst HPV-positive patients. There were four studies evaluating p53 with a total of 98 patients. Amongst HPV-positive patients, two studies (27,47) found that overexpression of p53 correlated with increased recurrence rates whilst two studies (43,46) found no correlation. Three studies evaluated ERCC1 with a total of 186 patients. Two studies (36,42) demonstrated no correlation between ERCC1 levels and recurrence. However, one study (31) demonstrated increased recurrence rates with higher expression of ERCC1. Two studies evaluated pRb with a total of 53 patients. One study (27) demonstrated increased recurrence rates with overexpression in pRb whereas one study (46) found no correlation. Two studies evaluated loss of ataxia telangiectasia mutated (ATM) with a total of 64 patients. One study (43) found that ATM loss correlated with increased recurrence rate whereas one study found no correlation (40). Correlations of other biomarkers evaluated in less than two studies can be found in Table 5.
Table 5
| Biomarker | Studies (n) | Patients (n) | % expressed | Summary |
|---|---|---|---|---|
| p53 | 4 | 98 | 16.1% | 2 studies—high p53 expression correlated with increased risk of recurrence: P=0.046, HR 0.31 (95% CI, 0.10–0.98) (Ryu et al., 2014); P<0.01, no HR reported (Fallai et al., 2009) |
| 2 studies—no correlation with recurrence: P=0.54, no HR reported (Chung et al., 2009); data not reported (Moeller et al., 2011) | ||||
| EGFR | 4 | 354 | 46.7% | 1 study—high EGFR expression correlated with increased risk of recurrence: P=0.02, no HR reported (Fei et al., 2009) |
| 3 studies—no correlation with recurrence: P=0.97, no HR reported (Husain et al., 2012); P=0.73, HR 3.84 (95% CI, 0.48–30.84) (Hong et al., 2010); P=0.205, HR 1.71 (95% CI, 0.75–3.87) (Vainshtein et al., 2014) | ||||
| ERCC1 | 3 | 186 | 38.7% | 2 studies—no correlation with recurrence: P=0.75, HR 0.7 (95% CI, 0.1–4.5) (Hao et al., 2010); P=0.138, HR 0.4 (95% CI, 0.1–1.3) (Song et al., 2013) 1 study—ERCC1 correlated with increased recurrence rates: ERCC1 (FL297): P=0.04, HR 10.1 (no 95% CI); ERCC1 (4F9): P=0.04, HR 13.7 (no 95% CI) (Bauman et al., 2013) |
| pRB | 2 | 53 | 11.3% | 1 study—low pRB associated with decreased risk of recurrence: P=0.01, HR 0.08 (95% CI, 0.02–0.35) (Ryu et al., 2014) |
| 1 study—no correlation with recurrence: P=0.54, no HR reported (Chung et al., 2009) | ||||
| ATM | 2 | 64 | 10.7% | 1 study—no correlation with recurrence (data not reported) (Lim et al., 2012) 1 study—ATM loss correlated with increased recurrence rate: P=0.03, no HR reported (Moeller et al., 2011) |
| ATR | 1 | 36 | NR | 1 study–ATR loss correlated with increased risk of recurrence: P=0.03, no HR reported (Moeller et al., 2011) |
| P21 | 1 | 23 | 78% | 1 study—no correlation with recurrence: P=0.43, no HR reported (Chung et al., 2009) |
| PD-1+ve cells | 1 | 32 | 59% | 1 study—no correlation with recurrence (data not reported) (Badoual et al., 2013) |
| CD163+ | 1 | 42 | 42.9% | 1 study—no correlation with recurrence: P=0.146, no HR reported (Balermpas et al., 2014) |
| CD11B+ | 1 | 42 | 53.6% | 1 study—no correlation with recurrence: P=0.418, no HR reported (Balermpas et al., 2014) |
| Ki67 | 1 | 48 | 56% | 1 study—Ki67 positivity inversely associated with recurrence: P=0.015, HR 0.21 (95% CI, 0.08–0.56) (Liu et al., 2014) |
| Cyclin D1 | 1 | 150 | 58.8% | 1 study—intensity of expression correlated with recurrence: P=0.014, no HR reported (Scantlebury et al., 2013) |
| VEGF | 1 | 42 | 59% | 1 study—no correlation with recurrence: P=0.4, HR 1.43 (95% CI, 0.63–3.53) (Fei et al., 2009) |
| SMG1 | 1 | 20 | 28% | 1 study—low SMG-1 correlated with decreased incidence of recurrence (no P value nor HR) (Gubanova et al., 2012) |
| 16q loss | 1 | 28 | 29% | 1 study—16q loss correlated with decreased rate of recurrence: P=0.008, no HR reported) (Klussmann et al., 2009) |
| 9p loss | 1 | 28 | 11% | 1 study—9p loss correlated with decreased rate of recurrence: P=0.04, no HR reported (Klussmann et al., 2009) |
| miR21 | 1 | 36 | 28% | 1 study—no correlation: P=0.564, no HR reported (Ko et al., 2014) |
| Ku80 | 1 | 36 | NR | 1 study—no correlation (data not reported) (Moeller et al., 2011) |
| E-cadherin | 1 | 36 | NR | 1 study—E-cadherin expression correlated with increased risk of recurrence: P=0.04, no HR reported (Moeller et al., 2011) |
| Bcl2 | 1 | 53 | 39.6% | 1 study—high Bcl2 correlated with higher risk of recurrence: P=0.004, HR 7.6 (95% CI, 1.9–30) (Nichols et al., 2010) |
| CDK4 | 1 | 46 | 43.5% | 1 study—high CDK4 associated with increased recurrence: P=0.009, HR 2.87 (95% CI, 2.51–3.46) (Park et al., 2012) |
| Heregulin | 1 | 68 | 47.4% | 1 study—no correlation with recurrence: P=0.309, no HR reported (Qian et al., 2015) |
| XPC rs2228000 | 1 | 102 | 47.1% | 1 study—SNP correlated with increased rate of recurrence: P=0.051, HR 1.6 (95% CI, 1.0–4.1) (Song et al., 2013) |
| XPC rs2228001 | 1 | 102 | 29.4% | 1 study—no correlation with recurrence: P=0.523, HR 0.7 (95% CI, 0.3–2.0) (Song et al., 2013) |
| XPA rs1800975 | 1 | 102 | 51.9% | 1 study—no correlation with recurrence: P=0.933, HR 1.0 (95% CI, 0.4–2.4) (Song et al., 2013) |
| XPD rs1799793 | 1 | 102 | 56.9% | 1 study—SNP correlated with increased rate of recurrence: P=0.002, HR 0.2 (95% CI, 0.1–0.5) (Song et al., 2013) |
| XPD rs13181 | 1 | 102 | 55.9% | 1 study—no correlation with recurrence: P=0.100, HR 0.4 (95% CI, 0.2–1.1) (Song et al., 2013) |
| XPG rs17655 | 1 | 102 | 31.4% | 1 study—SNP correlated with increased rate of recurrence: P=0.036, HR 0.1 (95% CI, 0.0–0.9) (Song et al., 2013) |
| LMP10 | 1 | 207 | 44.4% nucleus; 47.8% cytoplasm | 1 study—fraction of nuclear expression correlated with lower incidence of recurrence: P=0.009, HR 2.25 (95% CI, 1.35–7.85); cytoplasm expression did not correlated: P=0.093, HR 2.07 (95% CI, 0.89–4.84) (Tertipis et al., 2014) |
| TNF alpha-308 (rs1800629) GG | 1 | 158 | 63% | 1 study—GG genotype correlated with increased recurrence: P=0.005, HR 5.1 (95% CI, 1.4–18.4) (Zhang et al., 2014) |
| TNF alpha-857 (rs1799724) CC | 1 | 158 | 87% | 1 study—no correlation with recurrence: P=0.594, HR 1.4 (95% CI, 0.3–5.9) (Zhang et al., 2014) |
| TNF alpha-863 (rs1800630) CC | 1 | 158 | 46.8% | 1 study—CC genotype correlated with increased recurrence: P=0.007, HR 3.7 (95% CI, 1.5–9.1) (Zhang et al., 2014) |
| TNF alpha-1031 (rs1799964) TT | 1 | 158 | 69.6% | 1 study—no correlation with recurrence: P=0.100, HR 0.6 (95% CI, 0.2–1.3) (Zhang et al., 2014) |
| FAS1377 G>A GA+AA | 1 | 233 | 8.5% | 1 study—no correlation with recurrence: P=0.662, HR 0.8 (95% CI, 0.2–3.3) (Zhang et al., 2015) |
| FAS670 A>G AA+GG | 1 | 233 | 46.4% | 1 study—AG+GG mutation associated with increased risk of recurrence: P<0.0001, HR 12.9 (95% CI, 3.8–43.6) (Zhang et al., 2015) |
| FASLG844 C>T CC+TT | 1 | 233 | 35.2% | 1 study—AG+GG mutation associated with increased risk of recurrence: P<0.0001, HR 8.1 (95% CI, 3.6–18.6) (Zhang et al., 2015) |
| FASLG124 A>G AG+GG | 1 | 233 | 20.6% | 1 study—no correlation with recurrence: P=0.100, HR 1.6 (95% CI, 0.8–3.3) (Zhang et al., 2015) |
p53, protein 53; EGFR, epidermal growth factor receptor; ERCC1, excision repair cross-complementation group 1; pRB, retinoblastoma protein; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; p21, protein 21; PD-1, programmed cell death protein 1; CD, cluster of differentiation; VEGF, vascular endothelial growth factor; Ki67, marker of proliferation Ki-67; SMG1, suppressor with morphogenetic effect on genitalia; BCL2, B-cell Lymphoma 2; CDK4, cyclin dependent kinase 4; SNP, single nucleotide polymorphism; LMP10, low molecular weight protein 10; TNF-alpha, tumour necrosis factor alpha; FAS, Fas cell surface death receptor; FASLG, FAS ligand; HR, hazard ratio; CI, confidence interval.
Response to treatment
There were two studies evaluating response to treatment with one biomarker investigated in each study. ERCC1 correlated with better response to treatment (31) and SMG-1 negative tumours correlated with higher radiation sensitivity (38) (Table 6).
Table 6
| Biomarker | Studies (n) | Patients (n) | % expressed | Summary |
|---|---|---|---|---|
| ERCC1 | 1 | 56 | 35.7% | 1 study—ERCC1 (fl297): P=0.041; ERCC1 (4f9): P=0.016, correlated with better complete response (no HR reported) (Bauman et al., 2013) |
| SMG1 | 1 | 20 | 28% | 1 study—SMG1 negative tumours correlated with higher radiation sensitivity (no data values reported) (Gubanova et al., 2012) |
ERCC1, excision repair cross-complementation group 1; SMG1, suppressor with morphogenetic effect on genitalia; HR, hazard ratio; CI, confidence interval.
Distant metastasis
There were two studies evaluating rates of distant metastasis with four biomarkers investigated in total. Only NOTCH was found to be lower in patients developing distant metastasis (34) with p53, CD163 and CD11b showing no correlation (23,34) (Table 7).
Table 7
| Biomarker | Studies (n) | Patients (n) | % expressed | Summary |
|---|---|---|---|---|
| p53 | 1 | 15 | NR | 1 study—no correlation with metastasis: P=0.5, no HR reported (Kaka et al., 2013) |
| NOTCH | 1 | 15 | NR | 1 study—NOTCH lower in patients developing metastasis: P=0.04, no HR reported (Kaka et al., 2013) |
| CD163 | 1 | 42 | 42.9% | 1 study—no correlation with development of DM: P=0.140, no HR reported (Balermpas et al., 2014) |
| CD11b | 1 | 42 | 53.6% | 1 study—no correlation with development of DM: P=0.417, no HR reported (Balermpas et al., 2014) |
p53, protein 53; CD, cluster of differentiation; HR, hazard ratio; CI, confidence interval; NR, not reported.
Discussion
It is well described that particularly in the oropharyngeal literature, HPV-positive neoplasia represents a distinct clinical and biological entity from that of HPV-negative neoplasia. When functioning properly, p53 responds to cellular injury resulting in cell cycle arrest, attempted DNA repair, and, if DNA repair is ineffective, apoptosis. Mutations in p53 have been well established in HPV-negative SCC with prevalence in the literature to be between 47–100% (7,8,52). In this study, mutated p53 was prevalent in only 14% of HPV-positive cases. Overexpression of p53 was not found to be a reliable indicator of prognosis in this review with studies demonstrating mixed results. This contrasts with that of HPV-negative neoplasia in the literature in which p53 mutation has been demonstrated to be a poor prognostic indicator (53,54). EGFR activation induces cellular proliferation and prevents apoptosis. In the current review, EGFR overexpression showed 50% prevalence amongst HPV-positive patients, compared to more than 90% of HPV-negative or undifferentiated patients in the literature (55,56). Studies evaluating the prognostic value of EGFR showed mixed results in HPV-positive neoplasia, again contrasting the undifferentiated literature (55,56). Whilst there are a vast array of studies appraising prognostic biomarkers in UADT neoplasia, few studies differentiated between HPV-positive and negative tumours despite these essentially representing distinct pathologies. In this review, there were no biomarkers that reliably demonstrated prognostic value in HPV-positive tumours specifically in multiple studies.
All thirty-one included studies evaluated SCC of the UADT. There were no studies that met the inclusion criteria that evaluated prognostic biomarkers in HPV-positive benign neoplasia such as IP. Laryngeal papillomatosis is a relapsing remitting growth of the upper respiratory tract and is a benign manifestation of HPV. Recent studies have documented a high prevalence (20–50%) of laryngeal dysplasia in patients who have had a diagnosis of laryngeal papillomatosis (57-59). Whilst HPV has been implicated in the pathogenesis of IP and its malignant transformation to SCC, studies have not consistently demonstrated a true connection between the virus and IP nor whether or not HPV-positive IP behaves like HPV-positive malignant neoplasia, representing less aggressive disease (4-6).
Limitations in this analysis of the literature included the heterogeneity of the included studies such as differing primary modes of treatment, different population severity and variable follow-up periods. This restricts comparison of the studies and increases risk of confounding. Whilst there were many different biomarkers assessed in the various studies, few of these had more than one study to compare results. Meta-analysis was not considered appropriate due to the heterogeneity of populations, treatments and disease. Exclusion of studies that did not specifically examine HPV-positive neoplasia as distinct from HPV-negative neoplasia may have limited results, as the majority of studies identified in the search did not differentiate between these groups in their analysis.
Conclusions
It is well established that HPV-positivity correlates with improved prognosis in oropharyngeal SCC. However, there are no reliable biomarkers that can predict which tumours may fall into the more aggressive subset in this group. Further research is required to establish reliable prognostic biomarkers of HPV-positive SCC of the UADT. Furthermore, the influence of HPV on the behavioral or oncogenic influence in benign papilloma has yet to be fully defined.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: RH serves as the unpaid Editor-in-Chief of Australian Journal of Otolaryngology. R Sacks: Medtronic and Nycomed, consultant; Merck Sharp & Dohme and Arthrocare, speakers’ bureau. R Harvey: Medtronic, Olympus, and NeilMed Pharmaceuticals, consultant, with research grant funding received from Meda Pharmaceuticals and Stallergenes; GlaxoSmithKline and Arthrocare, speakers’ bureau. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [Crossref] [PubMed]
- Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011;333:1157-60. [Crossref] [PubMed]
- Lesperance MM, Esclamado RM. Squamous cell carcinoma arising in inverted papilloma. Laryngoscope 1995;105:178-83. [Crossref] [PubMed]
- Beck JC, McClatchey KD, Lesperance MM, et al. Human papillomavirus types important in progression of inverted papilloma. Otolaryngol Head Neck Surg 1995;113:558-63. [PubMed]
- Beck JC, McClatchey KD, Lesperance MM, et al. Presence of human papillomavirus predicts recurrence of inverted papilloma. Otolaryngol Head Neck Surg 1995;113:49-55. [Crossref] [PubMed]
- Habbous S, Pang V, Xu W, et al. Human papillomavirus and host genetic polymorphisms in carcinogenesis: a systematic review and meta-analysis. J Clin Virol 2014;61:220-9. [Crossref] [PubMed]
- Tabatabaeifar S, Kruse TA, Thomassen M, et al. Use of next generation sequencing in head and neck squamous cell carcinomas: a review. Oral Oncol 2014;50:1035-40. [Crossref] [PubMed]
- Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011;333:1154-7. [Crossref] [PubMed]
- Al-Swiahb JN, Huang CC, Fang FM, et al. Prognostic impact of p16, p53, epidermal growth factor receptor, and human papillomavirus in oropharyngeal cancer in a betel nut-chewing area. Arch Otolaryngol Head Neck Surg 2010;136:502-8. [Crossref] [PubMed]
- Broglie MA, Soltermann A, Rohrbach D, et al. Impact of p16, p53, smoking, and alcohol on survival in patients with oropharyngeal squamous cell carcinoma treated with primary intensity-modulated chemoradiation. Head and Neck 2013;35:1698-706. [Crossref] [PubMed]
- Duray A, Descamps G, Decaestecker C, et al. Human papillomavirus predicts the outcome following concomitant chemoradiotherapy in patients with head and neck squamous cell carcinomas. Oncology Reports 2013;30:371-6. [Crossref] [PubMed]
- Mullikin TC, Pasalic D, Garcia JJ, et al. Primary chemoradiation therapy versus surgery with adjuvant radiation therapy: Survival, failure rates, and toxicities for HPV positive oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2015;93:E318-9. [Crossref]
- Chowdhury N, Alvi S, Kimura K, et al. Outcomes of HPV-related nasal squamous cell carcinoma. Laryngoscope 2017;127:1600-3. [Crossref] [PubMed]
- Ganly I, Iyer G, Rahmati R, et al. Risk stratification based on HPV and smoking status independently predicts outcome in patients with oropharyngeal cancer treated with surgery and postoperative radiation. Int J Radiat Oncol Biol Phys 2014;88:475. [Crossref]
- Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol 2012;30:2102-11. [Crossref] [PubMed]
- Huang SH, Perez-Ordonez B, Liu FF, et al. Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:276-83. [Crossref] [PubMed]
- Sinha P, Thorstad WT, Nussenbaum B, et al. Distant metastasis in p16-positive oropharyngeal squamous cell carcinoma: a critical analysis of patterns and outcomes. Oral Oncol 2014;50:45-51. [Crossref] [PubMed]
- Sepiashvili L, Bruce JP, Huang SH, et al. Novel insights into head and neck cancer using next-generation "omic" technologies. Cancer Res 2015;75:480-6. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration 2011.
- Qian X, Wagner S, Ma C, et al. ALDH1-positive cancer stem-like cells are enriched in nodal metastases of oropharyngeal squamous cell carcinoma independent of HPV status. Oncol Rep 2013;29:1777-84. [Crossref] [PubMed]
- Zhang F, Sturgis EM, Sun Y, et al. Apoptotic variants as predictors of risk of oropharyngeal cancer recurrence after definitive radiotherapy. Int J Cancer 2015;137:2454-61. [Crossref] [PubMed]
- Balermpas P, Rodel F, Liberz R, et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br J Cancer 2014;111:1509-18. [Crossref] [PubMed]
- Kim MJ, Ki MS, Kim K, et al. Different protein expression associated with chemotherapy response in oropharyngeal cancer according to HPV status. BMC cancer 2014;14:824. [Crossref] [PubMed]
- Ko YH, Won HS, Sun DS, et al. Human papillomavirus-stratified analysis of the prognostic role of miR-21 in oral cavity and oropharyngeal squamous cell carcinoma. Pathol Int 2014;64:499-507. [Crossref] [PubMed]
- Liu J, Zhang M, Rose B, et al. Ki67 Expression has Prognostic Significance in Relation to Human Papillomavirus Status in Oropharyngeal Squamous Cell Carcinoma. Ann Surg Oncol 2015;22:1893-900. [Crossref] [PubMed]
- Ryu CH, Ryu J, Cho KH, et al. Human papillomavirus-related cell cycle markers can predict survival outcomes following a transoral lateral oropharyngectomy for tonsillar squamous cell carcinoma. J Surg Oncol 2014;110:393-9. [Crossref] [PubMed]
- Tertipis N, Haeggblom L, Nordfors C, et al. Correlation of LMP10 expression and clinical outcome in Human Papillomavirus (HPV) positive and HPV-Negative tonsillar and base of tongue cancer. PLoS One 2014;9:e95624. [Crossref] [PubMed]
- Vainshtein JM, Spector ME, McHugh JB, et al. Refining risk stratification for locoregional failure after chemoradiotherapy in human papillomavirus-associated oropharyngeal cancer. Oral Oncol 2014;50:513-9. [Crossref] [PubMed]
- Zhang C, Sturgis EM, Zheng H, et al. Genetic variants in TNF-alpha promoter are predictors of recurrence in patients with squamous cell carcinoma of oropharynx after definitive radiotherapy. Int J Cancer 2014;134:1907-15. [Crossref] [PubMed]
- Bauman JE, Austin MC, Schmidt R, et al. ERCC1 is a prognostic biomarker in locally advanced head and neck cancer: Results from a randomised, phase II trial. Br J Cancer 2013;109:2096-105. [Crossref] [PubMed]
- Chandarana SP, Lee JS, Chanowski EJ, et al. Prevalence and predictive role of p16 and epidermal growth factor receptor in surgically treated oropharyngeal and oral cavity cancer. Head Neck 2013;35:1083-90. [Crossref] [PubMed]
- Chiosea SI, Grandis JR, Lui VW, et al. PIK3CA, HRAS and PTEN in human papillomavirus positive oropharyngeal squamous cell carcinoma. BMC Cancer 2013;13:602. [Crossref] [PubMed]
- Kaka AS, Kumar B, Kumar P, et al. Highly aggressive human papillomavirus-related oropharyngeal cancer: clinical, radiologic, and pathologic characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:327-35. [Crossref] [PubMed]
- Scantlebury JB, Luo J, Thorstad WL, et al. Cyclin D1-a prognostic marker in oropharyngeal squamous cell carcinoma that is tightly associated with high-risk human papillomavirus status. Hum Pathol 2013;44:1672-80. [Crossref] [PubMed]
- Song X, Sturgis EM, Jin L, et al. Variants in nucleotide excision repair core genes and susceptibility to recurrence of squamous cell carcinoma of the oropharynx. Int J Cancer 2013;133:695-704. [Crossref] [PubMed]
- Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-Associated head and neck cancer. Cancer Res 2013;73:128-38. [Crossref] [PubMed]
- Gubanova E, Brown B, Ivanov SV, et al. Downregulation of SMG-1 in HPV-positive head and neck squamous cell carcinoma due to promoter hypermethylation correlates with improved survival. Clin Cancer Res 2012;18:1257-67. [Crossref] [PubMed]
- Husain H, Psyrri A, Markovic A, et al. Nuclear epidermal growth factor receptor and p16 expression in head and neck squamous cell carcinoma. Laryngoscope 2012;122:2762-8. [Crossref] [PubMed]
- Lim AM, Young RJ, Collins M, et al. Correlation of Ataxia-Telangiectasia-Mutated (ATM) gene loss with outcome in head and neck squamous cell carcinoma. Oral Oncol 2012;48:698-702. [Crossref] [PubMed]
- Park WS, Ryu J, Cho KH, et al. Human papillomavirus in oropharyngeal squamous cell carcinomas in Korea: use of G1 cycle markers as new prognosticators. Head Neck 2012;34:1408-17. [Crossref] [PubMed]
- Hao D, Lau HY, Eliasziw M, et al. Comparing ERCC1 protein expression, mRNA levels, and genotype in squamous cell carcinomas of the head and neck treated with concurrent chemoradiation stratified by HPV status. Head Neck 2012;34:785-91. [Crossref] [PubMed]
- Moeller BJ, Yordy JS, Williams MD, et al. DNA repair biomarker profiling of head and neck cancer: Ku80 expression predicts locoregional failure and death following radiotherapy. Clin Cancer Res 2011;17:2035-43. [Crossref] [PubMed]
- Hong A, Dobbins T, Lee CS, et al. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. Eur J Cancer 2010;46:2088-96. [Crossref] [PubMed]
- Nichols AC, Finkelstein DM, Faquin WC, et al. Bcl2 and human papilloma virus 16 as predictors of outcome following concurrent chemoradiation for advanced oropharyngeal cancer. Clin Cancer Res 2010;16:2138-46. [Crossref] [PubMed]
- Chung YL, Lee MY, Horng CF, et al. Use of combined molecular biomarkers for prediction of clinical outcomes in locally advanced tonsillar cancers treated with chemoradiotherapy alone. Head Neck 2009;31:9-20. [Crossref] [PubMed]
- Fallai C, Perrone F, Licitra L, et al. Oropharyngeal squamous cell carcinoma treated with radiotherapy or radiochemotherapy: prognostic role of TP53 and HPV status. Int J Radiat Oncol Biol Phys 2009;75:1053-9. [Crossref] [PubMed]
- Fei J, Hong A, Dobbins TA, et al. Prognostic significance of vascular endothelial growth factor in squamous cell carcinomas of the tonsil in relation to human papillomavirus status and epidermal growth factor receptor. Ann Surg Oncol 2009;16:2908-17. [Crossref] [PubMed]
- Klussmann JP, Mooren JJ, Lehnen M, et al. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin Cancer Res 2009;15:1779-86. [Crossref] [PubMed]
- Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol 2008;26:3128-37. [Crossref] [PubMed]
- Qian G, Jiang N, Wang D, et al. Heregulin and HER3 are prognostic biomarkers in oropharyngeal squamous cell carcinoma. Cancer 2015;121:3600-11. [Crossref] [PubMed]
- Gaykalova DA, Mambo E, Choudhary A, et al. Novel insight into mutational landscape of head and neck squamous cell carcinoma. PLoS One 2014;9:e93102. [Crossref] [PubMed]
- Bradford CR, Zhu S, Poore J, et al. p53 mutation as a prognostic marker in advanced laryngeal carcinoma. Department of Veterans Affairs Laryngeal Cancer Cooperative Study Group. Arch Otolaryngol Head Neck Surg 1997;123:605-9. [Crossref] [PubMed]
- Bossi P, Perrone F, Miceli R, et al. Prognostic role of PI3KCA and TP53 in HPV-negative oropharyngeal cancers (OPCs). J Clin Oncol 2011;29:5573. [Crossref]
- Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 2002;62:7350-6. [PubMed]
- Dassonville O, Formento JL, Francoual M, et al. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J Clin Oncol 1993;11:1873-8. [Crossref] [PubMed]
- Syrjanen K, Syrjanen S. Detection of human papillomavirus in sinonasal papillomas: systematic review and meta-analysis. Laryngoscope 2013;123:181-92. [Crossref] [PubMed]
- Blumin JH, Handler EB, Simpson CB, et al. Dysplasia in adults with recurrent respiratory papillomatosis: incidence and risk factors. Ann Otol Rhinol Laryngol 2009;118:481-5. [Crossref] [PubMed]
- Davids T, Muller S, Wise JC, et al. Laryngeal papillomatosis associated dysplasia in the adult population: an update on prevalence and HPV subtyping. Ann Otol Rhinol Laryngol 2014;123:402-8. [Crossref] [PubMed]
Cite this article as: Sacks PL, Alvarado R, Sacks R, Gallagher R, Harvey R. Prognostic biomarkers of human papilloma virus (HPV)-positive neoplasia of the upper aerodigestive tract: a systematic review. Aust J Otolaryngol 2018;1:14.