
Can sleep questionnaires predict outcome in children undergoing adenotonsillectomy for sleep disordered breathing?
Introduction
Paediatric sleep disordered breathing (SDB) encompasses a spectrum; ranging from the 14% of children who habitually snore, through to those with upper airways resistance syndrome (UARS), and finally to the 1–3% with obstructive sleep apnoea (OSA) where there is intermittent, complete airway obstruction (1). SDB is associated with adverse neurocognitive and physiological sequelae and successful treatment has been shown to improve both long-term social and cognitive potential in children (2-5).
One of the main causes of SDB in children is adenotonsillar hypertrophy and tonsillectomy +/− adenoidectomy (TA) is the most common, first-line treatment for SDB that can impact a child’s wellbeing (6). However, the pathogenesis of SDB is not solely based on adenotonsillar size. Contributions are also made by the tongue base, pharyngeal muscle tone, surrounding soft tissue bulk, and craniofacial structure (7). The complex aetiology of SDB makes it difficult to predict which child will benefit from TA and explains why clinical examination is seemingly poor at identifying children with OSA when compared to polysomnography (PSG) (8).
PSG is considered to be the gold standard of diagnosis for OSA. It assesses sleep related physiological variables and the resultant apnoea-hypopnoea index (AHI) is used to define OSA and its severity. However, using AHI as a measure of surgical outcome in the larger SDB group gives seemingly poor results. Previous studies have shown that TA will reduce the AHI in 79% of children with SDB (9) but only 60% will be normalised or “cured” (AHI <1) (10). Furthermore, PSG is time consuming, places a high burden on the child and family, and is not easily accessible in most centres.
In contrast, quality of life (QOL) questionnaire results have shown significant improvement in up to 95% of SDB children after TA (11-13). A variety of these questionnaires have been designed to assess SDB associated QOL. Despite being validated against PSG, they have a wide range of sensitivity and specificity for identifying those with OSA (14-16). Our study aims to assess the ability of sleep questionnaires to predict improvement following TA for children with SDB. Our hypothesis was that initial sleep questionnaire scores could be used to predict which children would most benefit from TA.
Methods
This is a case-control study. The primary aim was to assess the change in questionnaire score, following TA undertaken for SDB and to compare this to children who did not have SDB.
Participants
Case participants were eligible for inclusion if they were aged between 3 and 12 years at the time of recruitment and were on the surgical waiting list for adenotonsillectomy at Dunedin Public Hospital as treatment for adenotonsillar hypertrophy. Children had to live within the greater Dunedin area with access to a parent or guardian who was able to provide requested information. Children were excluded from participation if they displayed evidence of gross motor or sensory deficits, had significant medical comorbidity, or a diagnosis of Autism or psychoses. Control participants (without a documented history of snoring) were recruited through early childhood educators, caregivers and the Early Learning Project list (comprises children in the community whose caregivers consented to being contacted about research projects conducted in the University of Otago, Psychology Department). Children were enrolled in two overlapping studies assessing other aspects of SDB in childhood (SDB measurement and behaviour) and the number of children recruited into these studies dictated the sample size. Recruitment and relevant assessment methods for were identical for both studies. The University of Otago Human Ethics Committee gave ethical approval (H13/040 and H14/029) and informed consent was obtained from the caregivers of participating children prior to their inclusion into the study. The studies were conducted between October 2013 and March 2015.
Measures
Participant characteristics
At baseline, demographic and anthropometric data was collected which included age, sex, weight and height to calculate body mass index (BMI), ethnicity and deprivation index. BMI-for-age z-scores were calculated using WHO growth standards (17). Ethnicity was coded using a prioritized system where participants were assigned a single ethnic group using the following New Zealand Ministry of Health priority system (18): Māori (indigenous New Zealanders), Pacific Islander, Asian, other ethnicities (except NZ European), and NZ European. The New Zealand Deprivation Index (NZDep2013) (19) was used as an index of neighbourhood deprivation based on the participant’s address at baseline. This index ranges from 1 to 10, with 1 representing areas of least deprivation, and 10, areas of highest deprivation.
Questionnaires
Paediatric Sleep Questionnaire (PSQ)
The PSQ has 22 questions encompassing 4 domains: sleep related breathing, snoring, daytime somnolence, and behaviour. The rater indicates the presence or absence of the behaviour or symptom item by answering “yes”, “no” or “don’t know”. The answers are scored as “0” (symptom absent) or “1” (symptom present) and the overall score is calculated as a proportion of positive answers with a score greater or equal to 0.33 being considered predictive for SDB (20). For children aged 2–18 years, the scale has good internal consistency (α =0.88) with a sensitivity of 0.85 and specificity of 0.87 for identifying children with sleep related breathing disorders.
OSAS QOL Survey (OSA-18)
The OSA-18 has 18 questions encompassing 5 domains: sleep disorder, physical distress, emotional distress, diurnal problems, and caretaker preoccupation. Each item is rated for frequency of occurrence on a 7-point Likert scale by the caregiver. The scores on each of the 18 items are summed to produce a survey score ranging from 18 to 126 and a score greater or equal to 60 is considered predictive for SDB (21). Scores of <60 indicate a small impact on QOL, 60–80 indicate a moderate impact on QOL, and 80+ indicate a severe impact on QOL. All items in the scale have good test-retest reliability (r >0.74). The scale also demonstrates relatively good construct validity; OSA-18 scores have been correlated with the respiratory disturbance index on 90-minute nap PSG for children aged 6 months to 12 years (21).
Parents of enrolled children were asked to complete both questionnaires at least 3 weeks prior to surgery and then again between 3 and 7 months after surgery. Parents of the control group also filled out the questionnaires with a similar time interval.
Sleep studies
Children underwent a one-night Level 3 sleep study at baseline and at 3 months post-surgery, using an in-home portable device (Visi Black Shadow device; Stowood Scientific Instruments Ltd., UK). Recordings included pulse oximetry, ECG, nasal airflow and pressure, inductance plethysmography, snoring presence and loudness and actigraphy. Respiratory data were first scored automatically using the customized software excluding movement events indicative of waking. Respiratory events were then manually edited over 30 second epochs by a sleep researcher (blinded to patient information including testing sequence). The AHI was calculated according to the American Academy of Sleep Medicine (AASM) criteria for scoring paediatric respiratory sleep studies (22). An obstructive apneic event was defined as an airflow decrease ≥90% from the pre-event baseline for at least two respiratory cycles (2 breaths), with continued chest wall and abdominal wall movement. An obstructive hypopnea episode was defined as a decrease in nasal flow ≥30% (lasting at least 2 breaths), with a corresponding decrease in oxygen saturation ≥3% and at least one surrogate indicator of arousal (movement measured by actigraphy, changes in snoring sounds, >10% increase in heart rate or abrupt changes in thoracoabdominal effort) (23). A central apnoea was defined as a decrease ≥90% of the pre-event baseline measured by thermistor or a valid alternative sensor, lasting 20 seconds or at least the duration of two breaths and associated with a ≥3% oxygen desaturation or arousal indicated by surrogate measures as above, or lasts at least the duration of two breaths and is associated with a decrease in heart rate to <50 BPM for 5 seconds.
Statistical analyses
Data was analysed using Stata 15.1 (StataCorp, Texas, USA). Differences in baseline demographic and health characteristics between the surgical group and the control group were tested using either a t-test with unequal variances (age and BMI z-score) or Fisher’s exact test (sex and ethnicity). Differences in AHI, PSQ, and OSA-18 scores between groups were determined using t-tests with unequal variances, both before and after surgery. Mean differences between the groups and 95% confidence intervals (CI) were calculated, along with P values. Mean change in these scores after surgery (or after a similar time-frame in the control group) were calculated along with 95% CI. To determine if the change in scores was different between the surgical group and the control group, a t-test on the changes was undertaken. Additionally, to account for the very different baseline values between groups for each of the measures, a regression model for change in scores between groups with adjustment for baseline was also carried out.
As cut-off scores for PSQ (≥0.33) and AHI (≥1) are often utilised to assess whether SDB is present or absent, these were used to determine the number of children in the surgical group who had improved (score below the cut-off after surgery) or not improved (score above the cut-off after surgery). A McNemar’s test assessed whether one of these measures was more likely to detect improvement than the other. This was carried out in all surgical children, and then again only in the surgical children who were above the cut-off scores before surgery.
Results
Sixty-four children were included in the study, 45 in the surgical group and 19 in the control. While the initial questionnaires were completed for all children, 5 in the control group did not have follow-up questionnaire scores. When plotting change in questionnaire score over time (Figures 1-3) only children with scores at both time points are shown (surgical group n=40, control group n=19). Not all children underwent overnight PSG before and after surgery, 6 of the surgery group and 9 of the control group did not have the initial PSG and 3 of the surgery group and 11 of the control group did not have the second PSG. This did not reduce the number entered into the study, but influenced comparison of AHI with questionnaire scores.
There were no differences in demographics between the surgical and control groups at baseline (Table 1), although the surgical group had a slightly higher percentage of Māori children (20% compared to 11%).
Table 1
| Characteristics | Surgical group (n=45) | Controls (n=19) | P valuea for difference |
|---|---|---|---|
| Age (years), mean (SD) | 6.3 (2.5) | 6.4 (2.9) | 0.903 |
| Sex (male), n [%] | 21 [47] | 9 [47] | 0.959 |
| BMI z-score, mean (SD) | 0.9 (1.1) | 0.8 (0.9) | 0.588 |
| Ethnicity, n [%] | 0.779 | ||
| New Zealand European or similarb | 33 [73] | 16 [84] | |
| Maori | 9 [20] | 2 [11] | |
| Other or unknownc | 3 [7] | 1 [5] |
a, P values from t-test with unequal variances for continuous variables, and Fisher’s exact test for categorical variables; b, Australian, European, and American included; c, n=1 unknown, all others African, Indian, or Chinese.
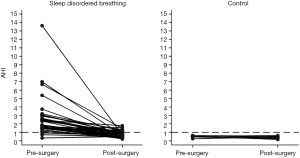
The PSQ and OSA-18 both scored significantly higher for the children with SDB prior to TA (Table 2). Following surgery, the scores were similar to the control group and there was a significant drop in the mean AHI, although it remained higher than for the control group. There was a significantly greater decrease in AHI, PSQ, and OSA-18 scores in the surgical group compared to the control group over the time when the surgical group had their TA (Table 3). However, after adjustment for baseline scores only the PSQ demonstrated significantly greater decreases in scores compared to the control group. Therefore, independent of baseline score, the PSQ was the most sensitive measure to detect improvement in SDB after TA. When the usual cut-offs were applied to the post-surgical data, both AHI and PSQ performed similarly in detecting improvement in SDB (Table 4).
Table 2
| Sleep disordered breathing measure | Control group, mean (SD) (n=19) | Surgical group, mean (SD) (n=45) | Mean difference (95% CI) | P value |
|---|---|---|---|---|
| Before surgery | ||||
| AHI | 0.7 (0.4) | 2.5 (2.3) | 1.8 (1.0, 2.6) | <0.001 |
| PSQ | 0.17 (0.10) | 0.55 (0.17) | 0.38 (0.32, 0.45) | <0.001 |
| OSA-18 | 31 [9] | 58 [14] | 27 [21, 33] | <0.001 |
| After surgery | ||||
| AHI | 0.4 (0.2) | 0.8 (0.4) | 0.3 (0.1, 0.5) | 0.002 |
| PSQ | 0.15 (0.14) | 0.20 (0.14) | 0.05 (−0.03, 0.12) | 0.247 |
| OSA-18 | 29 [9] | 30 [11] | 2 (−4, 7) | 0.550 |
AHI, apnoea-hypopnoea index; PSQ, paediatric sleep questionnaire.
Table 3
| Sleep disordered breathing measure | Mean change (95% CI) | Mean change compared to controls (95% CI) | P value | Mean change compared to controls, adjusted for baseline (95% CI) | P value |
|---|---|---|---|---|---|
| AHI control group (n=6) | −0.11 (−0.33, 0.11) | Reference | – | Reference | – |
| AHI surgical group (n=36) | −1.51 (−2.00, −1.02) | −1.40 (−1.92, −0.89) | <0.001 | 0.30 (−0.07, 0.66) | 0.106 |
| PSQ control group (n=19) | −0.02 (−0.07, 0.04) | Reference | – | Reference | – |
| PSQ surgical group (n=40) | −0.35 (−0.40, −0.30) | −0.33 (−0.41, −0.26) | <0.001 | −0.14 (−0.24, −0.04) | 0.007 |
| OSA-18 control group (n=19) | −3 (−6, 1) | Reference | – | Reference | – |
| OSA-18 surgical group (n=40) | −28 (−32, −23) | −25 (−32, −18) | <0.001 | −7 (−14, 0.5) | 0.069 |
AHI, apnoea-hypopnoea index; PSQ, paediatric sleep questionnaire.
Table 4
| Status according to AHI | Status according to PSQ | P valuea for difference between measures | |
|---|---|---|---|
| No improvement, n [%] | Improvement, n [%] | ||
| All children with sleep disordered breathing who had surgery and had AHI and PSQ measures before and after (n=33) | |||
| No improvement | 4 [12] | 7 [21] | 0.782 |
| Improvement | 6 [18] | 16 [48] | |
| Children with AHI score of at least 1 and PSQ score of at least 0.33 before surgery (n=26) | |||
| No improvement | 3 [12] | 4 [15] | 0.706 |
| Improvement | 3 [12] | 16 [62] | |
a, McNemar’s test. AHI, apnoea-hypopnoea index; PSQ, paediatric sleep questionnaire.
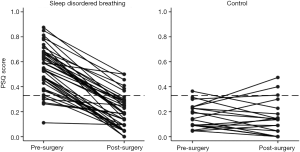
Figures 1 and 2 show the change in questionnaire score for each child with the cut-off score marked. Both graphs show that for most children undergoing TA for SDB there was an improvement in QOL score following surgery, whereas the control children showed little change in score. Considering the PSQ surgical group, 6 children scored below 0.33 preoperatively and 4 of these had minimal change in their postoperative score. There were also 6 children in the surgical group that remained above the 0.33 cut-off following surgery, although all but one had reduction in their scores. All the control children scored under 0.33 at their initial assessment; however, three scored above this level in the later assessment. The OSA-18 graph shows similar trends in scores but a larger proportion (52%) of the SDB group had a pre-operative score below the cut-off.
The raw data showed that 88% of children considered by clinicians to have SDB sufficient to warrant TA had a PSQ score >0.33; in contrast only 48% of the same children had a raised OSA-18. Among the SDB children with a PSQ score >0.33, 83% had a post-operative score below 0.33, whereas 45% of children with a pre-operative OSA-18 score >60 had a post-operative score of less than 60. Another way of looking at this is that 73% of children clinicians considered sufficiently symptomatic to warrant TA had a PSQ <0.33 following surgery, compared to 45% improved according to the OSA-18.
Discussion
A major dilemma in the management of paediatric SDB is the contrast between objective and subjective outcome measurements. PSG is the current gold standard for diagnosing OSA, the AHI accurately reflects its presence and can indicate severity (11). PSG is time consuming to perform and difficult to access. Our institution can only offer paediatric PSG as a research tool, which renders it clinically irrelevant as part of the assessment of children referred with symptoms of SDB. Furthermore, PSG does not quantify the impact that a specific AHI has on an individual’s QOL.
Kang et al. (10) reviewed 119 children undergoing AT for SDB, 93% had improvements to their OSA-18 score to below diagnostic cut-off, whereas only 54% had a postoperative AHI that was less than 1. This supports the opinion that AHI does not fully reflect the effect that SDB breathing may have on an individual. On the other hand, it may be argued that questionnaires are more open to bias and do not accurately reflect changes in symptoms.
Regardless of any perceived or actual bias, our results show that the PSQ has the potential to better identify children who will benefit from AT for SDB than the clinical assessment. The PSQ was originally developed by Chervin as a research alternative to PSG (20); scores above 0.33 being more strongly associated with an elevated AHI. Previous studies have shown the PSQ to have a sensitivity of 85–89% and a specificity of 41–87% for predicting an AHI >1 (1,24,25). Our results show that children with PSQ scores above 0.33 are likely to experience improved QOL following TA. They were also significant in that children who scored less than 0.33 pre-operatively did not show any benefit from TA. While a low pre-operative score inherently leaves less room for improvement, it suggests that the PSQ also has a negative predictive value. This is consistent with Chervin et al. (26) who reported that children with suspected SDB and low PSQ scores were more likely to self-resolve without surgical intervention.
The other questionnaire used in our study was the OSA-18. This was initially designed by Franco et al. (21) and validated using AHI derived from 90-minute nap PSGs after sleep deprivation the previous night. Multiple studies have shown that OSA-18 scores consistently improve after TA (3,27,28). However, when OSA-18 is compared to AHI there is variability in the reported sensitivity and specificity (11,15,16). Borgstrom et al. (14) found OSA-18 had a sensitivity of only 32% for identifying children with moderate to severe AHI, although specificity was 88%. The study also highlighted the potential inaccuracy of using nap rather than overnight oximetry. The authors concluded that whilst OSA-18 can document QOL, it has a high chance of missing children with severe SDB if used as a screening tool.
In contrast with both the PSQ and the AHI scores, over half of the pre-operative TA group scored below the OSA-18 cut-off, reducing the number of children who would have been recommended TA. Despite this all but one of the post-operative scores in the surgical group scored below the cut-off. These results are consistent with previous reports where OSA-18 scores improve significantly after TA (10,11,13). As an outcome measurement tool, the change in OSA-18 scores trended towards significance but was not as marked as the PSQ.
Among the control group most children showed little change in PSQ and OSA-18 score and this is to be expected as these are otherwise healthy children not suspected of having SDB. Some children showed an increased score at the second assessment; SDB is a dynamic condition and it is recognised that across childhood some children will cease snoring whereas others will develop snoring (29).
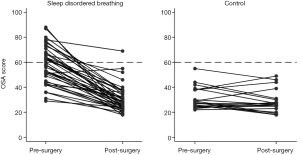
Our AHI data showed that the majority of the surgical group had OSA prior to their surgery, with 33% having AHI >1 post operatively. This is consistent with the results of a meta-analysis by Lee et al. (30) where they showed that residual OSA can be found in 51% of children after TA for SDB. A weakness in our study was that PSG was not performed on all participants and this limits our conclusions about the relationship between AHI and PSQ; for example, the sensitivity and specificity of PSQ as a predictor of AHI among our group.
A further limitation was the sample size, in particular the smaller number of children in the control group and those with AHI scores. However, estimates of the differences between the groups are presented, along with 95% CI, so that inferences do not need to rely solely on P values.
In summary, our study has shown that the PSQ may be used to identify otherwise healthy children, with sufficient SDB symptoms, that improved QOL may be expected following TA; and of equal importance, those who will not. As PSG is not easily accessible outside of major tertiary centres, we suggest that the PSQ could be used as an alternative tool to help identify children who will benefit from TA and prevent unnecessary surgery being carried out on those who will not. Further studies are needed to determine if these results are maintained in larger cohorts.
Acknowledgments
Funding: LA Smith was supported by the Linsell Richards Education Trust and the Freemasons of New Zealand Fellowship in Paediatrics. SE Maessen was supported by a University of Otago PhD Scholarship.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.01.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The University of Otago Human Ethics Committee gave ethical approval (H13/040 and H14/029) and informed consent was obtained from the caregivers of participating children prior to their inclusion into the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldstein NA, Pugazhendhi V, Rao SM, et al. Clinical assessment of pediatric obstructive sleep apnea. Pediatrics 2004;114:33-43. [Crossref] [PubMed]
- Galland BC, Dawes PJ, Tripp EG, et al. Changes in behaviour and attention capacity after adenotonsillectomy. Pediatr Res 2006;59:711-6. [Crossref] [PubMed]
- Ali NJ, Pitson D, Stradling JR. Sleep disordered breathing: effects of adenotonsillectomy on behaviour and psychological functioning. Eur J Pediatr 1996;155:56-62. [Crossref] [PubMed]
- Avior G, Fishman G, Leor A, et al. The effect of tonsillectomy and adenoidectomy on inattention and impulsivity as measured by the Test of Variables of Attention (TOVA) in children with obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 2004;131:367-71. [Crossref] [PubMed]
- Friedman BC, Hendeles-Amitai A, Kozminsky E, et al. Adenotonsillectomy improves neurocognitive function in children with obstructive sleep apnea syndrome. Sleep 2003;26:999-1005. [Crossref] [PubMed]
- Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med 2010;182:676-83. [Crossref] [PubMed]
- Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep 2004;27:997-1019. [Crossref] [PubMed]
- Brietzke SE, Katz ES, Roberson DW. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg 2004;131:827-32. [Crossref] [PubMed]
- Marcus CL, Moore RH, Rosen CL, et al. A randomised trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013;368:2366-76. [Crossref] [PubMed]
- Kang KT, Weng WC, Lee CH, et al. Discrepancy between objective and subjective outcomes after adenotonsillectomy in children with obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 2014;151:150-8. [Crossref] [PubMed]
- Baldassari CM, Alam L, Vigilar M, et al. Correlation between REM AHI and quality-of-life scores in children with sleep-disordered breathing. Otolaryngol Head Neck Surg 2014;151:687-91. [Crossref] [PubMed]
- Randhawa PS, Cetto R, Chilvers G, et al. Long term quality-of-life outcomes in children undergoing adenotonsillectomy for obstructive sleep apnoea: a longitudinal study. Clin. Otolaryngol 2011;36:475-81. [Crossref] [PubMed]
- Powell SM, Tremlett M, Bosman DA. Quality of life in children with sleep-disordered breathing treated with adenotonsillectomy. J Laryngol Otol 2011;125:193-8. [Crossref] [PubMed]
- Borgström A, Nerfeldt P, Friberg D. Questionnaire OSA-18 has poor validity compared to polysomnography in pediatric obstructive sleep apnoea. Int J Pediatr Otorhinolaryngol 2013;77:1864-68. [Crossref] [PubMed]
- Walter LM, Biggs SN, Cikor N, et al. The efficacy of the OSA-18 as a waiting list triage tool for OSA in children. Sleep Breath 2016;20:837-44. [Crossref] [PubMed]
- Ishman SL, Yang CJ, Cohen AP, et al. Is the OSA-18 predictive of obstructive sleep apnoea: comparison to polysomnography. Laryngoscope 2015;125:1491-5. [Crossref] [PubMed]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 2006;450:76-85. [PubMed]
- Ministry of Health. (2017b). HISO 10001:2017 Ethnicity Data Protocols. Available online: https://www.health.govt.nz/publication/hiso-100012017-ethnicity-data-protocols
- Atkinson J, Salmond C, Crampton P. Nzdep2013 Index of Deprivation. New Zealand, Ministry of Health, 2014.
- Chervin RD, Hedger K, Dillon JE, et al. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioural problems. Sleep Med 2000;1:21-32. [Crossref] [PubMed]
- Franco RA Jr, Rosenfeld RM, Rao M. First place--resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg 2000;123:9-16. [Crossref] [PubMed]
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597-619. [PubMed]
- Association AST. ASA Addendum to AASM guidelines for recording and scoring of Paediatric sleep. Blacktown, Australia: Australian Sleep Technologists Association, 2011.
- Kako H, Tripi J, Walia H, et al. Utility of screening questionnaire and polysomnography to predict postoperative outcomes in children. Int J Pediatr Otorhinolaryngol 2017;102:71-5. [Crossref] [PubMed]
- Kadmon G, Shapiro CM, Chung SA, et al. Validation of a pediatric obstructive sleep apnea screening tool. Int J Pediatr Otorhinolaryngol 2013;77:1461-4. [Crossref] [PubMed]
- Chervin RD, Ellenberg SS, Xiaoling Hou MS, et al. Prognosis for spontaneous resolution of OSA in children. Chest 2015;148:1204-13. [Crossref] [PubMed]
- Othman MN, See GB, Latif HA. Impact of adenotonsillectomy on the quality of life in children with sleep disordered breathing. Int J Pediatr Otorhinolaryngol 2016;91:105-7. [Crossref] [PubMed]
- Mohsen N, Susan A, Shahin B, et al. Sleep related quality of like before and after adenotonsilar surgery in pediatric population. Int J Pediatr Otorhinolaryngol 2014;78:330-3. [Crossref] [PubMed]
- Luo R, Schaughency E, Gill AI, et al. Natural history of snoring and other sleep-disordered breathing (SDB) symptoms in 7-year old New Zealand children: a follow up from age 3. Sleep Breath 2015;19:977-85. [Crossref] [PubMed]
- Lee CH, Hsu WC, Chang WH, et al. Polysomnographic findings after adenotonsillectomy for obstructive sleep apnoea in obese and non-obese children: a systematic review and meta-analysis. Clin Otolaryngol 2016;41:498-510. [Crossref] [PubMed]
Cite this article as: Chan BC, Galland BC, Smith LA, Maessen SE, Haszard JJ, Schaughency EA, Dawes PJD. Can sleep questionnaires predict outcome in children undergoing adenotonsillectomy for sleep disordered breathing? Aust J Otolaryngol 2019;2:6.


