
Delays to treatment initiation in the management of head and neck cancer
Introduction
Head and neck cancer accounts for 2.7% of all new cancer diagnoses and 2.4% of cancer deaths in Australia annually (1). The management pathway is resource intensive with multiple services to accommodate within a limited timeframe. Timely intervention is fundamental to curtail the destructive pathophysiology. Murphy et al. [2016] confirmed a correlation between prolonged treatment time and mortality (2). Through a multi-centre analysis of over 50,000 cases of head and neck squamous cell carcinoma (SCC), Murphy identified a statistically significant increase in mortality where treatment time exceeded 46–52 days and a further statistically significant mortality risk beyond 62–67 days. High case volume in large departments and time intensive pre-treatment investigations were factors contributing to prolonged treatment times.
Van Harten [2015] identified male gender, low socio-economic status, oropharyngeal primary and advanced stage as other factors associated with prolonged treatment time (3). Patel et al. [2012] reported that primary radiotherapy and invasive diagnostic procedures contributed to delays to treatment initiation (4).
There is no objective definition of what constitutes an acceptable time to treatment initiation (TTI) but the global consensus is to strive to reduce treatment times. Murphy et al. [2016] identified 46 and 62 days as mortality risk thresholds based on a definition from the time of most definitive diagnosis to initiation of curative therapy (2). In the United Kingdom, the NHS introduced the Cancer Plan, which mandated a maximum of 62 days from the time of first general practitioner referral to initiation of definitive therapy (5). The National Wait Time Strategy in Ontario defined cancer treatment targets based on disease severity triage scores (6). The Royal Adelaide Hospital Department of Otolaryngology Head and Neck Surgery is the primary provider of head and neck cancer services in South Australia. The Department services a large volume of disenfranchised patients requiring auspicious management of their disease. The department undertook a 12-month retrospective cohort study on treatment times to assess the functionality of the head and neck cancer management pathway. The aim of this research article was to identify and rectify preventable delays.
Methods
The Royal Adelaide Hospital Research Ethics Committee granted approval for this study. All new referrals to the Royal Adelaide Hospital Head and Neck outpatient department from January 1st to December 31st, 2015 were examined. The department cancer database was cross referenced to ensure all cases were identified. The inclusion criteria were new head and neck SCC managed with curative intent therapy. The selection criteria targeted general practitioner referrals exclusively. These referrals were identified as they were most likely to fully utilise all elements of the management pathway including diagnosis, planning and ancillary services. These cases would most accurately reflect the functionality of outpatient head and neck cancer management. Cases of recurrent SCC and referrals from another ENT clinician, inpatient department or emergency department were excluded due to selection bias. These cases were considered more likely to underutilise or take ‘short cuts’ through the treatment pathway due to pre-determined diagnoses, treatment plans or expedited access to inpatient resources.
The definition applied for time to treatment initiation (TTI) was the date of first outpatient ENT surgical consultation to the date of initiation of definitive treatment. This definition incorporated all components of the treatment timeline that the ENT clinician can influence.
Within this definition there were four key milestones: initial ENT surgical consultation, invasive diagnostic procedure, multi-disciplinary meetings (MDMs) and initiation of definitive treatment. Data was collected to generate individual patient timelines incorporating these milestones. Data was collected on patient demographics, imaging, ancillary appointments (including anaesthetic and peri operative physician), treatment modalities and treatment intent.
Data was analysed using Graphpad Prism Edition 7.05 software to calculate mean, P value and standard deviation. Surgical and non-surgical data sets were comparative analysed.
Results
There were 484 cases new referrals to the head and neck clinic from January 1st to December 31st 2015. Referrals included benign and malignant head and neck lesions, thyroid malignancies and laryngology pathology. There were 143 referrals suspicious for malignancy of which 94 were subsequently diagnosed with SCC. Curative treatment was commenced in 91 cases. These 91 cases formed the cohort. Surgery was the treatment modality in 75 cases (82.42%) and primary radiotherapy (+/− chemotherapy) was undertaken in the remaining 16 cases (17.58%). This distinction formed the surgical and non-surgical cohorts.
Time to treatment initiation
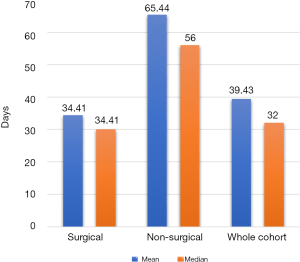
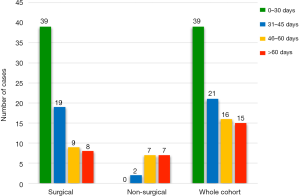
TTI was plotted for each patient from first ENT surgical consultation to initiation of treatment. The mean TTI for all cases (n=91) was 39.43 days with a standard deviation of 24.43 days (Figure 1). The surgical cohort (n=75) had a mean TTI of 34.41 days (standard deviation =20.66). The non-surgical cohort (n=16) had a mean TTI of 65.44 days (standard deviation =25.09). The TTI disparity between cohorts was statistically significant (P<0.001). All cases were subcategorised by length of TTI (Figure 2). 10.67% of surgical cases exceeded 60 days and 43.75% of non-surgical cases exceeded 60 days.
Diagnostic imaging
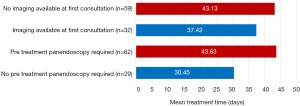
The availability of diagnostic imaging at first ENT surgical consultation was recorded. There was no available imaging during the first consultation in 32 cases (35.16%). Patients without imaging during the initial consultation had a mean TTI of 43.13 days. Comparatively, patients with imaging had a shorter mean TTI of 37.42 days. Simplistically, this 5.71-day differential reflected the time required for new imaging to be arranged and interpreted (Figure 3).
Invasive diagnostic procedures
Panendoscopy, examination under anaesthesia and microlaryngoscopy were categorised as invasive diagnostic procedures. These procedures were undertaken in 62 cases (68.13%). Patients that required an invasive diagnostic procedure (n=62) had a mean TTI of 43.63 days compared with 30.45 days when diagnostic procedures were not required (n=29). The 13.18-day TTI differential crudely reflected the time and resources required to coordinate diagnostic procedures under anaesthetic (Figure 3).
Ancillary services
Of the 62 cases undergoing invasive diagnostic procedures, 50 were later treated with surgery. This necessitated two general anaesthetics within a short interval. One outpatient anaesthetic appointment was deemed adequate in 18 of 50 cases (36%). The remaining 32 cases (64%) were scheduled a second outpatient anaesthetic encounter within an already heavily burdened timeline.
Within the surgical cohort, 21 patients (28%) attended a physician review for medical optimisation prior to surgery. In 7 cases, this was scheduled to coincide with the anaesthetic appointment. The remaining 14 cases were scheduled on a separate date. This data highlights the advantage of efficient scheduling to maximise patient convenience and minimize treatment times.
Investigation and planning
Head and neck cancer management requires a combination of investigation and planning. Investigation is defined as the interval from first ENT consultation to time of definitive diagnosis (diagnostic procedure). The key elements of this portion of the timeline are scheduling imaging and diagnostic procedures. The mean investigation interval was 18.3 days.
The planning interval is defined from the time of diagnosis (diagnostic procedure) to initiation of treatment. In the UK under the NHS Cancer Plan, this is referred to as the ‘decision to treat’ interval. The NHS mandates a maximum of 31 days within the ‘decision to treat’ interval (5). Within this cohort (n=62), the mean ‘decision to treat’ or planning interval was 26 days (20.3 days for surgical and 42.7 days for non-surgical).
Overall the planning interval (26 days) was 42% longer than the investigation interval (18.3 days). MDMs are the key milestone in the planning interval. They allow all disciplines to discuss and formalise treatment recommendations. Pre-treatment MDMs were utilised in the majority of cases (n=64). The mean interval from MDM to treatment initiation was 23 days (surgical 20 days, non-surgical 36 days). In 5 cases, there were two MDMs held prior to treatment initiation. This was in the setting of restaging due to concurrent physical illness or failure to attend follow up. Patient’s that required two MDMs (n=5) had a mean TTI of 61.4 days.
Discussion
The aim of this study was to assess the efficiency of the outpatient head and neck cancer pathway. The main outcome was isolating patient and treatment factors associated with prolonged treatment times. Concurrent physical illness, failure to attend follow up, inadequate imaging at initial consultation, time intensive diagnostic procedures and non-surgical treatment were factors associated with prolonged treatment times. The purpose of identifying these factors was to introduce targeted strategies to reduce TTI and improve patient outcomes.
The mean TTI disparity between surgical (34.4 days) and non-surgical treatment (65.4 days) was statistically significant (P<0.001). The referral pathway into the institution is predominantly via the ENT surgical service. Therefore, referrals to non-surgical disciplines, typically, are not made until after MDM discussion. Non-surgical treatment has resource intensive milestones (simulation scans, mask fittings, dental clearances) which need to be considered when examining this disparity. The disparity may also reflect a degree of selection bias. Patients deemed unfit for anaesthetic are more likely to be recommended non-surgical treatments. These patients are more prone to treatment interruptions secondary to concurrent illness. The prolonged TTI in the non-surgical cohort strongly emphasises the critical importance of communication. Establishing an efficient, transparent referral avenue between surgical and non-surgical departments is critical to reduce unacceptable treatment times.
The absence of diagnostic imaging at first ENT surgical consultation was a factor contributing to prolonged TTIs. Patients attending their first consultation without imaging (TTI 43.13 days) waited 6 days longer for treatment than those attending with imaging (TTI 37.42 days). Scheduling appropriate imaging to ensure results are available at the initial consultation would eliminate the observed treatment delay. Introducing a cancer coordinator to screen referrals and organise imaging would remedy this undue interruption.
Tissue diagnosis is fundamental for treatment planning. Panendoscopy, microlaryngoscopy and examination under anaesthesia achieve this through an invasive approach. A significant portion of the cohort (68.13%) underwent an invasive diagnostic procedure prior to treatment with a mean TTI of 43.6 days. Comparatively, when such procedures were not indicated the mean TTI was 30.45 days. Invasive diagnostic procedures are not without their risks. With advancements in imaging and office-based technology, a dependency on anaesthetic based procedures may become less significant. The department has reviewed its practice in this regard to identify contemporary indications for pre-treatment examination under anaesthesia (7). This strategy is aimed at maintaining best practice medicine and diagnostic yield whilst optimising treatment times and patient safety.
Conclusions
Head and neck cancer is a complex journey for patient and clinician. The destructive pathophysiology necessitates efficacious treatment. This data has identified patient and treatment factors that can compromise treatment times. The key findings were that concurrent physical illness, failure to attend follow up, oversaturation of ancillary appointments, inadequate imaging at initial consultation, time intensive diagnostic procedures and non-surgical treatment are all associated with prolonged treatment times.
Many treatment delays can be mitigated through simple and cost-effective initiatives. Routinely highlighting ‘time since referral’ into MDM presentations would ensure treatment time is considered in all clinical discussions. Assigning a case manager to each patient would foster individual accountability and awareness of treatment times. Developing a tick box checklist at clinical encounters would provide a tangible and visible representation of treatment times. Such practical initiatives can be easily integrated. They encourage proactive intervention when delays are encountered and act as a recurring stimulus for efficacious treatment.
Investing in a cancer coordinator would ensure referrals are prioritised, imaging is contemporary, ancillary services are streamlined and transition of care between departments is efficient. Most critically, cancer coordinators become a critical interface between the disenfranchised patient and the clinician.
Invasive diagnostic procedures play an integral role in specific indications but carry a procedural risk and prolong treatment times. Refining the indications for these procedures can improve patient safety provided diagnostic yield is maintained. Modern techniques like office based trans-nasal biopsies could become low risk alternatives that reduce dependency on more invasive options.
Head and neck cancer is a destructive pathology responsible for significant morbidity and mortality. Identifying strategies to reduce treatment time is critical to curtail this burden. The introduction of improved communication avenues, care coordination and innovative diagnostic techniques will ensure auspicious treatment is delivered to a vulnerable patient population.
Acknowledgments
This research was presented at the Australian Society of Otolaryngology, Head and Neck Surgery (ASOHNS) Annual Scientific Meeting held in Adelaide, Australia 23–26 March 2017 and at the International Federation of ORL Societies (IFOS) ENT World Congress held in Paris, France 24–28 June 2017.
Funding: None
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2020.02.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was granted by the Royal Adelaide Hospital Human Research Ethics Committee (HREC). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ho W, Shukla S, Mills L, et al. Cancer in Australia: an overview 2012. Australian Institute of Health and Welfare & Australasian Association of Cancer Registries 2012;74:70. ISBN: 978-1-74249-386-2.
- Murphy CT, Galloway TJ, Handorf EA, et al. Survival Impact of Increasing Time to Treatment Initiation for Patients With Head and Neck Cancer in the United States. J Clin Oncol 2016;34:169-78. [Crossref] [PubMed]
- van Harten MC, Hoebers FJ, Kross KW, et al. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol 2015;51:272-8. [Crossref] [PubMed]
- Patel UA, Brennan TE. Disparities in head and neck cancer: assessing delay in treatment initiation. Laryngoscope 2012;122:1756-60. [Crossref] [PubMed]
- Department of Health. NHS Cancer Plan. Available online: https://www.thh.nhs.uk/documents/_Departments/Cancer/NHSCancerPlan.pdf
- Cancer Care Ontaria. Wait times for cancer surgery in Ontario. Available online: https://www.cancercareontario.ca/en/content/target-wait-times-cancer-surgery-ontario
- Noor A, Stepan L, Kao SS, et al. Reviewing indications for panendoscopy in the investigation of head and neck squamous cell carcinoma. J Laryngol Otol 2018;132:901-5. [Crossref] [PubMed]
Cite this article as: Connell JT, Sekhar V, Hodge JC, Krishnan S, Foreman A. Delays to treatment initiation in the management of head and neck cancer. Aust J Otolaryngol 2020;3:5.


