
The accuracy of lateral X-ray and computed tomography in diagnosis of paediatric retropharyngeal abscess: a systematic review
Introduction
Retropharyngeal abscess (RPA) is an uncommon but potentially life-threatening paediatric emergency presentation. Prompt clinical suspicion, investigations and management are required to mitigate complications that can range from mediastinitis to acute airway compromise (1-3). Adjunct diagnostic modalities include lateral radiograph (XR) and computed tomography (CT). Despite substantial radiological advancements over several decades, the accuracy and indication for each modality in the workup of RPA remains somewhat unclear with no consensus guidelines within the literature. Certainly, XR was the initial diagnostic tool of choice as a non-invasive, widely available, cheap and sensitive investigation (4-6). However, recent literature has challenged the accuracy and merit of the XR given the availability of more advanced diagnostic aids (7-9). CT provides three-dimensional (3D) anatomical information that may be more sensitive in detecting RPA and help in surgical planning and performance (6,10-12). However, CT lacks specificity (6,7,13), exposes children to radiation (14,15) and therefore requires careful consideration when being used in the diagnosis of paediatric RPA. To add to the confusion, several authors suggest that certain RPAs may respond to intravenous antibiotics alone (without operative drainage), and therefore it may be suitable to adopt a ‘watch and wait’ approach that avoids any imaging investigations unless clinical deterioration dictates a need (6,10). As it stands, there are no consensus guidelines or algorithm for the investigation and management of RPA.
This systematic review was designed to address some of these questions, specifically the utility (if any) and accuracy of XR and CT in the investigation and management of paediatric RPA. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/ajo.2020.03.02).
Methods
Eligibility criteria
Inclusion and exclusion criteria were predefined. The final selection included English language, human studies with patients younger than 18 years of age that analysed either CT or XR in paediatric RPA comparing findings to operative findings. Articles analysing but not separating parapharyngeal/other deep neck space abscesses from RPA were excluded. Despite having a close anatomical/clinical relationship, these entities were not included as they may alter the perceived sensitivity, specificity and accuracy of the XR that measures retropharyngeal width.
Diagnostic modalities
Any articles that compared XR and/or CT findings to intraoperative outcomes were included for analysis. Ultrasound, magnetic resonance imaging (MRI), endoscopy, fluoroscopy or any other forms of imaging were deemed outside the scope of this review.
Outcome measures
The primary outcome was measured as the efficacy of XR and CT in predicting intraoperative pus. This was either defined as sensitivity, specificity, PPV and/or NPV.
Other demographic factors gathered included clinical findings, laboratory findings, microbiological isolate, type of management (medical or surgical), length of in-hospital stay and complications (Table 1).
Table 1
| Study, y | Clinical findings | Laboratory | Bacterial | Medical management | Surgical management | |||
|---|---|---|---|---|---|---|---|---|
| LOS (days) | Complications | LOS (days) | Complications | |||||
| Martin et al., 2014 | Fever, neck pain, torticollis | Leukocytosis | Streptococcus | NR | NR | NR | NR | |
| Nazir et al., 2013 | Fever, neck pain, dysphagia, odynophagia | NR | Staphylococcus, Streptococcus, Klebsiella | NR | Failed antibiotic treatment [3] | NR | Recurrence [2] | |
| Hoffman et al., 2011 | Fever, pain, neck stiffness | Leukocytosis | Streptococcus | 4.45 | Failed antibiotic treatment [8] | 4.75 | Recurrence [9] | |
| Pharisa et al., 2009 | Fever, neck swelling, limited neck movement | NR | NR | NR | NR | NR | NR | |
| Craig et al., 2004 | Neck pain, fever, odynophagia | NR | Streptococcus | 3.2 | Nil | 5.1 | Nil | |
| Stone et al., 1999 | NR | NR | N/A | NR | NR | NR | Recurrence [2] | |
| Boucher et al., 1999 | NR | NR | N/A | NR | NR | NR | NR | |
| Choi et al., 1997 | NR | NR | N/A | NR | NR | NR | NR | |
| Ravindranath et al., 1993 | NR | NR | Streptococcus | NR | NR | NR | NR | |
| Glasier et al., 1992 | NR | NR | N/A | NR | NR | NR | NR | |
| Coulthard et al., 1991 | Fever, stridor, neck swelling | NR | Staphylococcus | NR | NR | NR | Recurrence [6] | |
| Yeoh et al., 1985 | Neck stiffness, feeding difficulties, drooling, cervical swelling, fever, stridor | NR | Staphylococcus, Streptococcus, Klebsiella | NR | NR | NR | Recurrence [5] | |
NR, not recorded; LOS, length of stay.
Search strategy
A systematic search was performed using the PubMed, MEDLINE and EMBASE databases. The PubMed database was searched from inception until February 10, 2019; EMBASE was searched from 1974 to February 10, 2019, and MEDLINE was searched from 1946 to February 10, 2019 using Ovid SP. Bibliographies of studies selected for full-text analysis were cross referenced for any additional missing studies. An electronic search strategy was designed to identify all studies comparing lateral XR, CT and intraoperative findings in paediatric RPA.
Relevant studies were found using search terms “retropharyngeal abscess”, “computed tomography”, “CT”, “x-ray”, “xray”, “radiograph” and “radiography”.
Data collection and analysis
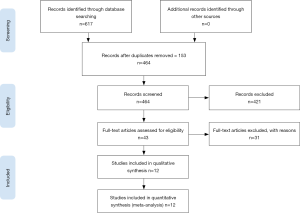
Two unblinded authors (R Daniel, P Stokes) reviewed all titles, abstracts, read full-text articles and compared them with predetermined inclusion criteria. Studies that met the inclusion criteria had the relevant data extracted using a standardised data form. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for study selection (Figure 1). The review authors conducted the data extraction and assessed the quality of methodology of each included trial. Considered factors were:
- Number of participants;
- Age of participants;
- Sociodemographic data;
- Characteristics of study;
- Inclusion and exclusion criteria;
- Risk of bias;
- Diagnostic criteria;
- Timing of investigations and operative management;
- Treatment:
- Intravenous antibiotic administration.
- Operative drainage.
- Follow up period;
- Adverse effects.
Risk of bias
Risk of bias for cohort studies was assessed in accordance with the Newcastle-Ottawa Scale (16).
Results
Search strategy
A total of 617 references were identified through the applied search strategy. First level screening removed 574 studies (duplicates, non-English, clearly irrelevant scope) leaving 43 references for full text consideration. A further 31 publications were excluded because they did not meet the predefined inclusion criteria. Twelve articles were chosen for final review (Table 2). Of these included papers that compared radiological findings to operative findings in paediatric RPA, six analysed CT, one analysed XR, and five analysed a combination of XR and CT.
Table 2
| Study, y | Method | Total participants | XR participants | CT participants | Age (median, mean or range) | Sex (male:female ratio) | Co-morbidities recorded |
|---|---|---|---|---|---|---|---|
| Martin et al., 2014 | Retrospective | 18 | 0 | 18 | 3.2 years old (median) | 1.6:1 | NR |
| Nazir et al., 2013 | Prospective | 57 | 57 | 57 | 0–15 years old (range) | 1.7:1 | NR |
| Hoffman et al., 2011 | Retrospective | 101 | 0 | 99 | 4.3 years old (mean) | 2:1 | Yes |
| Pharisa et al., 2009 | Retrospective | 3 | 1 | 3 | 9 years old (median) | 1.8:1 | NR |
| Craig et al., 2004 | Retrospective | 64 | 43 | 64 | 3 years old (mean) | NR | NR |
| Stone et al., 1999 | Retrospective | 34 | 0 | 34 | 3 months – 9 years old (range) | NR | NR |
| Boucher et al., 1999 | Retrospective | 25 | 24 | 15 | 0–15 years old (range) | NR | Yes |
| Choi et al., 1997 | Retrospective | 12 | 0 | 12 | 3.4 years old (mean) | 1.8:1 | NR |
| Ravindranath et al., 1993 | Retrospective | 10 | 10 | 10 | 4 months – 12 years old (range) | 1.5:1 | NR |
| Glasier et al., 1992 | Retrospective | 11 | 11 | 10 | 1–11 years old (range) | 1.8:1 | NR |
| Coulthard et al., 1991 | Retrospective | 31 | 24 | 0 | 6 days – 12 years old (range) | 1.2:1 | Yes |
| Yeoh et al., 1985 | Retrospective | 16 | 9 | 0 | Less than 6 years old (range) | 2.8:1 | NR |
NR, not recorded.
Demographics
The age of children included in this review ranged from 4 months (12) to 15 years (6,17), with a male predominance documented in all papers that recorded sex (4,5,8,9,11,12,17). The comorbidities of children were infrequently documented (6,8) (Table 2).
The most common clinical features of paediatric RPA were neck pain, fever and swallowing problems (4,7-9,17), with the most common microbiological isolates being gram positive cocci (streptococcus and staphylococcus species) (4,7,8,10,12,17,18) (Table 1).
Measurements
A lateral neck radiograph was used as standard imaging for XR in all studies (4-6,12,17). The most common radiological features for diagnosis of RPA was the width of retropharyngeal soft tissue (4-6,12,17). Retropharyngeal soft tissue was compared to adjacent vertebral bodies based off historical parameters (19) in three papers (4,5,12). The specific definition of abnormal varied from 50 (17) to 200 percent (4) the width of the adjacent vertebral body, with one paper not defining an abnormal limit (6). Additional findings suspicious for abscess included air fluid levels (4,17), gas or visible pre-vertebral shadow (4,6,17) and straightening of normal cervical lordosis (17).
One of the pitfalls found in this study is the lack of information and homogeneity in regards to the imaging equipment and technique. Only Ravindranath et al. (12) commented on the type of scanner used (General Electronic Healthcare 8800), adding to potential variability in outcomes. Only Glassier et al. (5) and Ravindranath et al. (12) noted the use of 5 mm interval slices and Choi stating 3 mm intervals in the majority of patients. No other studies commented on CT interval size. Four of the articles reviewed mention the use of contrast (5,11-13), with the remaining studies not reporting on whether contrast was used or not.
The landmark study by Wholey et al. (19) and Seid et al. (20) were referenced in certain studies to state the well established radiological definitions for RPA when using lateral XR (4,6,13). The strongest predictive sign stated by the Boucher et al. (6) study was retropharyngeal air, with all patients positive for RPA with this finding.
Six studies described CT characteristics of RPA, correlating a rim enhancing lesion with low attenuation centrally (5,6,8,10,13). However, Stone et al. (13) states these findings are predictive but not definitive for RPA. To differentiate an abscess from phlegmon, it is shown a phlegmon will show “obliteration of fat planes and oedema of the soft tissues” (13). Craig et al. (10) referenced Kirse and Roberson (21) who found a higher sensitivity if there was scalloping of the abscess wall. Hoffman et al. (8) established that the greatest sensitivity and specificity were rim enhancement and that a core density less than 32 Hounsfield units best correlates with a true RPA (8). Eight of the nine studies utilising CT mention the significant diagnostic and prognostic value of CT (5,6,8,10-13,17), however, four mention the lack of clear objective radiological criteria (5,8,10,13).
Study outcomes
All studies compared radiological findings with intraoperative pus as the gold standard. The sensitivity and specificity of XR ranged from 0 (12) to 100 percent (4-6) (Table 3). CT sensitivity ranged from 69 (8) to 100 percent (5,6,9,11,12) with a more variable specificity of 45 (6) to 100 percent (8,12) (Table 3). Overall, CT had a less variable sensitivity, specificity, PPV and NPV compared to XR (Table 3). In addition, one paper noted performance of surgery was significantly enhanced by CT (10) whilst three papers overtly noted CT to be helpful for surgical planning (6,11,12).
Table 3
| Study, y | CT | XR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||
| Martin et al., 2014 | 92 | 50 | 79 | 75 | NR | NR | NR | NR | |
| Nazir et al., 2013 | NR | NR | 83 | NR | NR | NR | 70 | NR | |
| Hoffman et al., 2011 | 69 | 100 | NR | NR | NR | NR | NR | NR | |
| Pharisa et al., 2009 | 100 | NR | 67 | NR | NR | NR | NR | NR | |
| Craig et al., 2004 | NR | NR | NR | NR | NR | NR | NR | NR | |
| Stone et al., 1999 | 81 | 50 | 84 | 44 | NR | NR | NR | NR | |
| Boucher et al., 1999 | 100 | 45 | 40 | 100 | 80 | 100 | 100 | 94 | |
| Choi et al., 1997 | 100 | 81 | 75 | 100 | NR | NR | NR | NR | |
| Ravindranath et al., 1993 | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Glasier et al., 1992 | 100 | 50 | 30 | NR | 100 | NR | 27 | NR | |
| Coulthard et al., 1991 | NR | NR | NR | NR | 88 | NR | NR | NR | |
| Yeoh et al., 1985 | NR | 100 | NR | NR | 100 | NR | 100 | NR | |
NR, not recorded.
Risk of bias
Eleven of the included studies were retrospective case series. The Newcastle-Ottawa Scale was applied to all included papers, deeming the majority to be low to poor quality (Table 1). Duration of symptoms at the time of presentation was infrequently reported (4,8,17), as was documentation regarding prehospital treatment (4,10,13,17). The time from imaging to operative drainage was recorded in two papers (12,13). Seven articles had historical imaging reviewed by a radiologist (5-7,10-13). The radiologist was blinded in two studies (7,11).
Discussion
Historically XR was seen as the gold standard for diagnosing paediatric RPAs (4-6). XR is appealing as it is a quick and easy test to perform, widely accessible and therefore is often seen as an ideal ‘screening test’. The landmark papers of Wholey et al. (19) and Seid et al. (20) defined the anatomical measurements for normal retropharyngeal width in the paediatric population. These papers also noted the importance of attaining a proper lateral neck radiograph and correct interpretation. False positives arise for a plethora of reasons including incorrect rotation, neck extension and/or respiratory phase as well as normal anatomical variance of cervical lordosis. This combined with an unwell, agitated child make the lateral XR prone to error (10,12).
Despite this, high sensitivity and specificity rates were documented within these studies: Yeoh et al. stated 100 percent sensitivity; Glasier et al. stated 100 percent sensitivity; Boucher et al. stated 80 percent sensitivity and 100 percent specificity; Coulthard et al. stated 88 percent sensitivity (Table 3). The authors therefore recommended XR as the ideal imaging modality for diagnosis. However, in the current systematic review, the lateral XR showed significant variance from 0 to 100 percent highlighting the inherent issue with this test. Papers that showed higher sensitivity and specificity had populations that tended to be severely unwell. Complication rates were high, with documentation of abscesses ‘self-discharging’ in emergency departments, tracheostomy insertion and two deaths (5,6,18) all suggestive of severe clinical presentation. In one paper (4), sensitivity was improved when retropharyngeal width was twice that of the adjacent cervical vertebra [i.e., twice as wide as what was originally deemed abnormal by Wholey et al. (19)] further emphasizing how large these abscesses were. It is difficult to conclude this as the most sensitive XR characteristic given most studies stated different opinions in relation to vertebral width. Compounding this, certain papers showed both false positive and false negative results (10,12).
In clinical practice, a combination of clinical features and certain XR characteristics may increase the diagnostic sensitivity, however there was not sufficient data in any paper correlating these two factors to determine this. With these factors in mind, it is difficult to validate the utility of such a diagnostic test in the screening of paediatric RPA.
CT remains the gold standard for diagnosis of paediatric RPA but is not without its pitfalls. Technological advances over several decades have allowed CT to become an efficient, accessible and economic diagnostic modality. It is intuitive that 3D anatomical imaging would outperform two-dimensional (2D) radiography for diagnostic accuracy (5,6,9,11,12), operative planning (guiding intraoral or transcervical approaches) and overall surgical performance (6,10-12). Although CT remains a highly sensitive diagnostic modality being able to delineate radiological cellulitis from an abscess (5,6,9,11,12), its variable specificity (6,7,13) may lend itself to unnecessary operations. Certain radiological features such as size of abscess, scalloped margins and rim enhancement may improve specificity and the likelihood of positive findings of pus at the time of surgery (21) and certainly some clinical practice guidelines agree with this (22). Interestingly, none of the included papers truly account for the natural history of suppuration and the bearing that time has on radiological and operative findings. Naturally, lymph nodes will take time to suppurate and a CT performed too early may over call a positive diagnosis (6); yet if clinical suspicion is high, investigations should not be unnecessarily delayed for this potentially life threatening entity. The severity and duration of a child’s symptoms in addition to the timing of antibiotic administration, diagnostic imaging and surgery are likely to affect intraoperative findings. The wary clinician should consider all these factors prior to ordering radiology to help better delineate which children will likely require operative management, and which children will respond to medical management alone thus avoiding unnecessary imaging and radiation exposure.
This review highlights several limitations inherent within the analysed literature. Firstly, without submitting all children with clinical suspicion of RPA to imaging (namely CT) and operative drainage, it is impossible to deduce true sensitivity and specificity and to do so would carry serious ethical, clinical and economic implications. With the overall lack of data (Table 3) and inability of comparison between each study patient group and their interventions, there was not sufficient information to perform a meta-analysis. A modified measurement of accuracy (i.e., CT in predicting pus at the time of surgery) based on retrospective data remains problematic, as there will still be a proportion of children whom respond to intravenous antibiotics that have radiological evidence of an RPA (10,11). Secondly, papers rarely controlled or accounted for confounding variables including the severity of clinical presentation, timing of antibiotic administration and imaging and the subsequent timeframe to theatre, all of which have a significant bearing on the likelihood of pus at the time of surgical drainage. Finally, the current data is largely taken from small retrospective case series that are underpowered. Several of the included studies suffered from selection and recall bias, and given that authors and/or additional assessors (radiologists) were often not blinded to outcomes, the veracity of their findings could be questioned. Furthermore, no consensus guidelines exist for the diagnosis and management of paediatric RPA and therefore the timing of investigations and management may vary depending on clinician preference which makes quantitative analysis difficult.
Conclusions
Current data validates CT as the gold standard in the diagnosis of RPA, but remains far from a perfect test. Lateral XR may be useful if stringent reporting criteria were developed and adhered to but it cannot guide surgical management. The informed clinician should understand the pitfalls associated with radiology in the diagnosis of paediatric RPA, and be aware of clinical findings that make CT more likely to be a useful diagnostic adjunct. The authors propose early ENT consultation to guide initial medical management. If initial clinical signs of severity are high or are worsening then the recommended primary imaging modality is CT. This will ultimately guide surgical management. Ideally larger longitudinal studies that account for the clinical severity (at the time of presentation), timing of investigations and time to surgery may help better inform consensus guidelines/protocols that can be widely applied to emergency, paediatric and ENT surgeons alike for the diagnostic and treatment pathway for paediatric RPA.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/ajo.2020.03.02
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2020.03.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tebruegge M, Connell T, Curtis N. Tuberculosis in children. N Engl J Med 2012;367:1568-author reply 9. [Crossref] [PubMed]
- Philpott CM, Selvadurai D, Banerjee AR. Paediatric retropharyngeal abscess. J Laryngol Otol 2004;118:919-26. [Crossref] [PubMed]
- Morrison JE, Pashley NR. Retropharyngeal abscesses in children: a 10-year review. Pediatr Emerg Care 1988;4:9-11. [Crossref] [PubMed]
- Yeoh LH, Singh SD, Rogers JH. Retropharyngeal abscesses in a children's hospital. J Laryngol Otol 1985;99:555-66. [Crossref] [PubMed]
- Glasier CM, Stark JE, Jacobs RF, et al. CT and ultrasound imaging of retropharyngeal abscesses in children. AJNR Am J Neuroradiol 1992;13:1191-5. [PubMed]
- Boucher C, Dorion D, Fisch C. Retropharyngeal abscesses: a clinical and radiologic correlation. J Otolaryngol 1999;28:134-7. [PubMed]
- Martin CA, Gabrillargues J, Louvrier C, et al. Contribution of CT scan and CT-guided aspiration in the management of retropharyngeal abscess in children based on a series of 18 cases. Eur Ann Otorhinolaryngol Head Neck Dis 2014;131:277-82. [Crossref] [PubMed]
- Hoffmann C, Pierrot S, Contencin P, et al. Retropharyngeal infections in children. Treatment strategies and outcomes. Int J Pediatr Otorhinolaryngol 2011;75:1099-103. [Crossref] [PubMed]
- Pharisa C, Lutz N, Roback MG, et al. Neck complaints in the pediatric emergency department: a consecutive case series of 170 children. Pediatr Emerg Care 2009;25:823-6. [Crossref] [PubMed]
- Craig FW, Schunk JE. Retropharyngeal abscess in children: clinical presentation, utility of imaging, and current management. Pediatrics 2003;111:1394-8. [Crossref] [PubMed]
- Choi SS, Vezina LG, Grundfast KM. Relative incidence and alternative approaches for surgical drainage of different types of deep neck abscesses in children. Arch Otolaryngol Head Neck Surg 1997;123:1271-5. [Crossref] [PubMed]
- Ravindranath T, Janakiraman N, Harris V. Computed tomography in diagnosing retropharyngeal abscess in children. Clin Pediatr (Phila) 1993;32:242-4. [Crossref] [PubMed]
- Stone ME, Walner DL, Koch BL, et al. Correlation between computed tomography and surgical findings in retropharyngeal inflammatory processes in children. Int J Pediatr Otorhinolaryngol 1999;49:121-5. [Crossref] [PubMed]
- Brenner D, Elliston C, Hall E, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001;176:289-96. [Crossref] [PubMed]
- Brady Z, Forsythe AV, Mathews JD. The changing use of pediatric CT in Australia. Pediatr Radiol 2016;46:1199-208. [Crossref] [PubMed]
- Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane. 2019. 2019.
- Nazir KA, Fozia PA, Ul Islam M, et al. Paediatric acute retropharyngeal abscesses: an experience. Afr J Paediatr Surg 2013;10:327-35. [Crossref] [PubMed]
- Coulthard M, Isaacs D. Retropharyngeal abscess. Arch Dis Child 1991;66:1227-30. [Crossref] [PubMed]
- Wholey MH, Bruwer AJ, Baker HL. The lateral roentgenogram of the neck; with comments on the atlanto-odontoid-basion relationship. Radiology 1958;71:350-6. [Crossref] [PubMed]
- Seid AB, Dunbar JS, Cotton RT. Retropharyngeal abscesses in children revisited. Laryngoscope 1979;89:1717-24. [Crossref] [PubMed]
- Kirse DJ, Roberson DW. Surgical management of retropharyngeal space infections in children. Laryngoscope 2001;111:1413-22. [Crossref] [PubMed]
- Philpott C, Huston K. Retropharyngeal abscess. In: BMJ Best Practice. BMJ Publishing Group, London. 2019. 2019.
Cite this article as: Daniel R, Stokes P, Dhillon K, Walsh P. The accuracy of lateral X-ray and computed tomography in diagnosis of paediatric retropharyngeal abscess: a systematic review. Aust J Otolaryngol 2020;3:12.


