
Inverted papilloma of the temporal bone: recent experience and a systematic review of management
Introduction
Inverted papilloma (IP) is an uncommon benign neoplasm with malignant potential arising from the Schneiderian Membrane (1), and has been occasionally reported to involve the temporal bone (2).
The first reported case of IP involving the temporal bone by Stone et al. was in 1987 (3). Since that time there has been a slow accumulation in reported cases (2). Although progress has been made in understanding this disease, its rarity has meant only a basic appreciation of this disease and its management is known. A number of theories regarding the aetiology of temporal bone IP (IPTB) have been proposed; including transmission through or invasion along the Eustachian Tube, ectopic Schneiderian Membrane within the middle ear that undergoes transformation to IP, or squamous metaplasia of middle ear mucosa secondary to chronic inflammation, eventually leading to IP formation (3,4). IPTB has been differentiated into primary (PIPTB) and secondary (SIPTB) types, but there exists no consensus on how to define these subtypes and whether this classification is of use.
The management of sinonasal IP has evolved to a point where there is now a well-defined treatment approach, with endoscopic surgery the favoured option (5-7). In addition to surgery there are adjuvant therapies such as radiation therapy or chemotherapy which may be used in certain situations such malignant transformation (7). However, the optimal management of IPTB is still uncertain and poorly defined within the sparse literature regarding this entity. This paper reviews two cases of IPTB and details their management. A literature review focusing on the management of IPTB is then presented with the aim of better defining this rare disease and the approach to treatment. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/ajo-20-74).
Methods
Ethics approval for this case series was granted by the Institution’s Ethics Review Board. Two cases of IPTB were identified, which were managed from 2015 until the present day. The details of these cases and their management are described.
In addition, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used to perform a systematic review of the current literature (8). The objectives for the systematic review were to compare the outcomes of surgical and non-surgical management of patients with inverted IPTB and to assess if clinicopathologic factors influenced these outcomes. A literature review was performed on 25th June 2020 of Medline, EMBASE, and The Cochrane Library. The search strategy aimed to include all articles concerning IPTB or subsites. Two main search domains were used which were combined with the Boolean operator “AND”, while search terms contained within each domain were combined with the Boolean operator “OR”. The first search domain included “Inverted Papilloma” or “Inverting Papilloma”. The second search domain included “Temporal Bone”, “Mastoid”, or “Middle Ear”.
Studies were selected if they met the following inclusion criteria; (I) English language, (II) pathologically confirmed IPTB or subsites. Studies were excluded if they contained insufficient detail for analysis. The search results were reviewed for eligibility and if the title or abstract suggested a potentially eligible study, whether abstract or full paper, was assessed by a single reviewer. The references were then checked to identify any other potentially eligible studies.
The primary outcome of the literature review was to determine the treatment types (surgery, radiation, chemotherapy) and outcomes (disease-free survival, disease specific survival) for IPTB, and the secondary outcomes were demographic (gender, age, location) and clinicopathologic aspects [primary or secondary IPTB, malignancy, human papilloma virus (HPV) status] of IPTB. Risk of bias was also assessed using a standardised tool (9).
There is no clear definition in the literature of PIPTB or SIPTB. In this review, PIPTB is defined as IPTB occurring solely in the temporal bone without any previous or concurrent IP in the sinonasal cavity, and SIPTB is defined as IPTB occurring in the setting of previous or current sinonasal IP. Surgery with a curative intent, aiming for an R0 or R1 resection, was classified broadly as either conservative or radical. Conservative surgery we defined as any resection less extensive than a modified radical mastoidectomy, while radical surgery was defined as a modified radical mastoidectomy or more extensive ablation. We defined tumour debulking as surgery where the incomplete removal was the likely and intended outcome, resulting in an R2 resection. Typically, tumour debulking was a limited procedure where only gross disease was extirpated. We defined salvage surgery (either conservative or radical) as revision surgery performed with curative intent after failed initial conservative or radical surgery.
The included studies were all retrospective case reports or series. Time from diagnosis to progression was calculated, and patients who did not progress were censored at the last date of follow-up when no progression was confirmed. The Kaplan-Meier method was used to calculate disease free-survival probabilities. The survival analysis for disease-free survival was based on initial surgery with curative intent. The data was considered not suitable for meta-analysis due to heterogeneity of treatment and likelihood of bias in the published studies. The database was compiled into Excel version 16.6.11 (Microsoft Corp., Redmond, WA, USA) for Mac (Cupertino, CA, USA) and analysed using SPSS version 26 (IBM, Armonk, NY, USA).
Case 1
A 55-year-old male initially presented in late 2014 with symptoms associated with a left nasal mass and ipsilateral middle ear effusion. He had no relevant background medical history. A magnetic resonance imaging (MRI) scan performed in December 2014 showed a soft tissue mass involving the left maxillary, ethmoid and sphenoid sinuses with fluid signal in the left middle ear and mastoid but no extension of the mass into the postnasal space or middle ear cleft. A biopsy at this time revealed IP with carcinoma in situ (CIS).
In January 2015 endoscopic resection of the sinonasal lesion was performed and also insertion of a left tympanostomy tube. During placement of the tympanostomy tube a middle ear polyp was discovered and biopsied. Pathology from both the sinonasal lesion and middle ear polyp revealed a mixture of both exophytic and IP with no malignant features.
Post-operative MRIs performed in 2015 showed post-surgical changes in the sinuses and a mass in the left middle ear cleft extending to the Eustachian tube and tegmen tympani with adjacent dural enhancement. Subsequently a cortical mastoidectomy was performed revealing extensive disease with in the middle ear and mastoid with involvement of the dura. The case was discussed in a Head and Neck Cancer Multi-Disciplinary Meeting (MDM) with the consensus for close observation, in light of the patient’s preference for a conservative approach and low chance of complete tumor removal due to the degree of dural involvement with IP. A surveillance MRI in October 2017 showed no recurrence of the sinonasal tumour but progression of the IPTB with disease involving the external auditory canal, middle ear cleft, mastoid, eustachian tube and dura but without intracranial extension.
Transcanal debulking procedures were performed in 2018 and in 2020. This involved removing only the IP that was protruding through a tympanic membrane perforation and extending down the external auditory canal. The rationale was to reduce otorrhoea and visible tumour while minimising surgical risk in a tumour that was behaving indolently. Histopathology continued to show dysplasia but without malignant transformation. The patient was also keen to explore non-surgical treatments for IP, and so underwent vaccination with Gardasil® (10).
Case 2
A 66-year-old male initially presented in late 2017 with symptoms associated with a nasal mass. His background medical history included type 2 diabetes treated with oral hypoglycaemics. Initial MRI and computed tomography (CT) imaging showed a lesion in the posterior nasal cavity extending into the nasopharynx and biopsy confirmed mixed inverted and exophytic papilloma with CIS. Repeat CT and MRI in 2018 showed bilateral middle ear and mastoid effusion without any bone erosion. The patient was consented for an endoscopic resection of the sinonasal IP and tympanostomy tube insertion, but was reluctant to undergo surgery and so this was not performed until October 2018. Intra-operatively polypoid masses were identified in the both middle ear clefts, and these were biopsied. The histopathology from the sinonasal tumour and the middle ear polyps, showed IP which was similar to the initial biopsy. The patient refused further treatment at this stage so was placed under active surveillance.
In late 2019 the patient was referred to our institution for management of his bilateral IPTB and deteriorating hearing. Examination revealed IP fungating down his external auditory canals bilaterally but no sinonasal recurrence. Repeat MRI showed progression of the IPTB to involve both eustachian tubes and with bilateral tegmen erosion and enhancement of the brain parenchyma on the left. Despite this the patient opted for ongoing observation, however the patient was keen to explore non-surgical treatment options, and agreed to undergo vaccination with Gardasil® (10).
In February 2020 the patient presented with a progressive right facial nerve palsy. Repeat MRI was performed and this showed no significant interval change in disease or radiologic evidence of perineural tumour spread. A right sided tympanomastoidectomy and blind sac was performed to assess for malignant transformation. Pathology demonstrated basaloid squamous cell carcinoma (SCC) arising from IP, with immunohistochemical staining positive for p16 and p40 and negative for CK7, while the Ki67 was low. The case was discussed at a Head and Neck Cancer MDM and the recommendation was for radiation therapy, however the patient opted for ongoing observation and there has been gradual disease progression on imaging.
Results
The literature search yielded a total of 128 potential articles suitable for analysis. All duplicates were removed, as a number of published reports involve the same case of IPTB. Abstracts of the remaining articles were reviewed to check they met inclusion and exclusion criteria. The full text of eligible articles were then assessed. Of the 128 potential articles 85 were excluded leaving 43 suitable for the review. The reference of the 43 studies were then hand searched for further eligible articles resulting in inclusion of an additional seven studies. As a result, there were 50 articles (Table 1) appropriate for inclusion in the review accounting for 55 cases, and with inclusion of the cases in this article there were a total of 57 cases of IPTB for analysis. The cases of IPTB include both PIPTB and SIPTB. Demographics are outlined in Table 2.
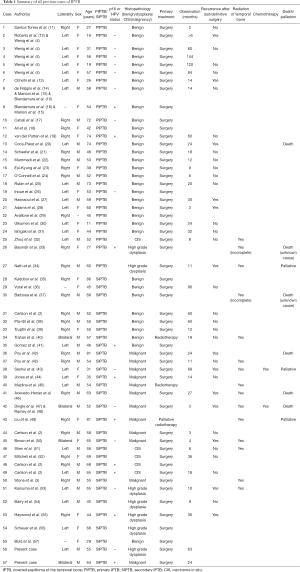
Full table

Full table
IPTB
Overall, for both PIPTB and SIPTB (n=57), the mean age was 53.4 years (SD: ±16.3) and the male to female ratio was 1:0.9. The ratio of right to left ears was 1:1 and there were four cases of bilateral disease. There were 23 cases tested for HPV or p16 status and 43.5% (n=10) of those tested were positive. Fifty-seven point nine percent (n=33) of cases were classified as benign while 42.1% (n=24) were dysplastic or malignant.
PIPTB
There were 27 cases of PIPTB. The average age was 49.4 years (SD: ±18.1). The male to female ratio was 1:1.6. The ratio of right to left ears was 1.1.4 and there were no cases of bilateral disease. Of the 11 cases tested for HPV or p16, 27.2% (n=3) were positive. Eighty-eight point nine percent (n=24) of cases were benign, while 8.3% (n=2) of cases had some degree of dysplasia and 4.2% (n=1) of cases were malignant.
With respect to the management of PIPTB (Table 3), there was one case where the type of surgery was not specified. Initially, conservative surgery was performed in 13 cases and debulking surgery was carried out in three cases. Of these, one case had no outcomes reported, six cases had no recurrence and nine cases had recurrence or residual disease. Six recurrences were after conservative surgery. Further salvage surgery was performed in six cases after failure of initial conservative surgery, of these one was salvaged with conservative surgery, and five were salvaged with radical surgery, with one dying shortly after radical surgery.

Full table
Of the cases initially treated with radical surgery (n=10), one case had no reported outcomes, two cases had recurrence, and there was no recurrence in seven cases. Of the two cases that recurred, one was palliated and the other died of disease.
The timing of recurrence after surgery for PIPTB was inconsistently reported but most recurrences were within 2 years.
SIPTB
There were 30 cases of SIPTB. The average age was 57.0 years (SD: ±13.7). The male to female ratio was 1:0.6. The ratio of right to left ears was 1:0.7 and there were four cases of bilateral disease. There were 12 cases tested for HPV or p16 and 58.3% (n=7) of cases tested were positive. Thirty percent (n=9) of cases were benign and 16.7% (n=5) of cases had dysplasia, all high grade, and 53.3% (n=16) of cases were malignant.
With respect to the management of SIPTB (Table 3), three cases did not have surgery.
Initial treatment was debulking surgery in three cases and conservative surgery in six cases. Of these, two recurred, three had residual disease, three reported no recurrence and one outcome was not reported. The two recurrences were after initial conservative surgery. Salvage treatment was recommended in the two cases that failed initial conservative surgery; one was treated with conservative surgery and the other with radiation.
There were 18 cases initially treated with radical surgery, with seven recurring, nine with no recurrence, and one with no reported outcome. Of the seven cases with recurrence, one refused radiotherapy and died, one was only able to tolerate part of the radiotherapy regime before dying, three were salvaged with further surgery and radiotherapy with only two surviving, and two patients were given further surgery, radiotherapy and chemotherapy, with one surviving and the other not.
Recurrence after surgery for SIPTB was inconsistently reported but most recurrences were seen within 1 year.
Outcomes
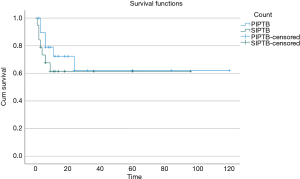
The mean disease-free survival (Table 4) for IPTB is 76.3 months (SD: ±10.1), while the mean disease free-survival for PIPTB is 78.6 months (SD: ±14.1), and the mean disease-free survival for SIPTB is 60.6 months (SD: ±10.6). A Kaplan-Meyer analysis was performed and the survival plot is shown in Figure 1. A log-rank test for equality of survival distributions showed no significant difference (P=0.498) between disease recurrence after initial treatment for PIPTB and SIPTB.

Full table
Benign IPTB
Benign IPTB was found in 57.9% (n=33) of cases, with 14 initially treated with conservative surgery, four treated with debulking surgery, 13 treated with radical surgery, in one case the type of surgery was not specified, and one patient declined surgery and was treated with radiotherapy.
When considering only definitive treatment where there was surgery with a curative intent (conservative or radical surgery), there were seven failures and five underwent surgical salvage. Two patients died of progressive benign IPTB.
Malignant or dysplastic IPTB
In 42.1% (n=24) of cases there was some degree of dysplasia or malignancy on histopathology (Table 5). Dysplasia, when found, was for the majority cases classified as severe. Initial treatment for the 24 cases included seven treated with less than radical surgery, 15 treated with radical surgery, one patient refused surgery and had radiation, and another received palliative radiotherapy.

Full table
If only cases with treatment with definitive or curative intent were considered then ten cases had recurrence. Four cases were salvaged, one patient with radiation alone, one with surgery alone, and two with a combination of radiation and surgery. Four patients died of their disease and another three were described as being palliated.
In addition, PIPTB had 88.9% (n=24) of cases classified as benign, 7.4% (n=2) as dysplastic and 3.7% (n=1) were malignant or CIS. While for SIPTB, which is a histologically more aggressive disease, 30% (n=9) were classified as benign, 16.7% (n=5) as dysplastic (high grade), and 53.3% (n=16) as malignant or CIS.
Human papilloma virus
HPV positivity for PIPTB was 27.2% (n=3) and for SIPTB was 58.3% (n=7), however only 40.4% (n=23) of cases of IPTB were analysed for HPV or a surrogate marker (Table 5).
Non-surgical therapy
Non-surgical therapy such as chemotherapy or radiation therapy was used in select patients and most commonly as an adjuvant treatment. Radiation therapy was given to 26.3% (n=15) of patients in total, and 3.5% (n=2) of patients received chemotherapy (Table 3). Of those that received radiation, in 13 cases it was for malignant or dysplastic disease and in two cases for benign disease; the latter comprised of one patient who refused surgery opting instead for radiation and the other patient diagnosed with recurrent disease. Chemotherapy was only used in the treatment of SIPTB for recurrent malignant disease. Of the 15 cases of IPTB that received radiation three were in PIPTB and 12 were in SIPTB.
Discussion
Due to the rarity of IPTB there has only been 55 unique cases reported in the literature to date. This has generated only a basic understanding of this disease, and as a result there has been limited evolution in the management of IPTB. Currently, there is no consensus on how to differentiate PIPTB from SIPTB and whether this distinction is valid. In this review, PIPTB is defined as IP occurring solely in the temporal bone or a subsite without any previous or concurrent IP in the sinonasal cavity. It has been posited that PIPTB can occur synchronously or metachronously with sinonasal IP when no direct connection, either clinically or radiologically, is found between two lesions but instead as a result of multifocal disease. Multifocal IP, occasionally reported in the literature, is difficult to prove conclusively as a cause of PIPTB (7). As seen in both cases described here, initially no direct connection was evident radiologically between the IP in the sinonasal cavity and the IPTB, but over time, disease became radiologically apparent along the eustachian tube. It is possible that this is a result of collision between multifocal IP, but more likely reflects a single tumour where the connection between two seemingly distinct parts is actually clinically and radiologically occult early in the course of the disease. Trying to determine a direct connection between IP in the sinonasal cavity and the temporal bone is difficult due to anatomic limitations that prevent direct examination and because low volume mucosal disease may be below the resolution of imaging. In addition, as discussed by Carlson et al. [2015], IPTB tends to be found ipsilateral to any sinonasal IP, making the argument for multifocality seem less plausible (2). It has been suggested that a delay in occurrence between sinonasal IP and IPTB may also indicate multifocal disease (35,51,58), but on the other hand delayed recurrence of sinonasal IP is not atypical. For sinonasal IP, the literature indicates the majority of recurrences occur within 2 years but 17% occur after 5 years and 6% after 10 years, necessitating long term follow-up (59). Therefore, we define SIPTB as IPTB occurring in the setting of any previous or current sinonasal IP.
The true rate of SIPTB is difficult to determine, in part due to inconsistencies defining and reporting PIPTB and SIPTB, and also due to the fact sinonasal IP that has undergone malignant transformation with resulting skull base or temporal bone invasion may be mistakenly interpreted as SIPTB (2,32,42).
Overall, IPTB presents on average at 53.4 years with an almost even sex distribution, however, there are differences in clinical presentation between PIPTB and SIPTB. PIPTB presents at a younger age and with a female preponderance. Four cases of bilateral disease were found in SIPTB while none in PIPTB, possibly reflecting a more aggressive disease process.
The rate of dysplasia and malignancy in SIPTB (70.0%) was much higher than in PIPTB (11.1%). Whether SIPTB is an innately more aggressive or the histopathology reflects aggressive sinonasal IP that progresses to directly involve the temporal bones is unclear. Certainly, the latter scenario seems plausible, however this does not fully account for the 30% of SIPTB cases with a benign histopathology.
There were higher rates of HPV positivity seen in SIPTB (58.3%) than PIPTB (27.2%). However, only 40.4% (n=23) of cases of IPTB were analysed for HPV or surrogate marker, with testing rates low and inconsistently performed. The relationship between sinonasal IP and HPV is unclear, but evolving (15,60-63). So too is the relationship between HPV and IPTB and its contribution to subsequent malignant transformation requires further investigation (15).
The treatment for IPTB has not been as clearly defined as it has for sinonasal IP (6,64). However, 78% (n=39) of published papers analysed in this review recommended complete surgical excision of the disease of IPTB as first line management. Conservative surgery was more likely to be attempted in PIPTB (50%) than in SIPTB (22.2%); and radical surgery more likely to be performed in SIPTB (66.7%) than PIPTB (38.5%). It is difficult to compare surgical outcomes between PIPTB and SIPTB, as surgical approaches that were utilised varied widely within the broad categories of conservative and radical surgery. The extent of surgery tended to reflect the extent and nature of the disease, with radical surgery generally reserved for advanced disease, malignant disease or SIPTB, often with adjuvant therapy added for malignant change or incomplete resection.
An estimate of failure or disease recurrence after initial surgical treatment considering only those cases undergoing curative intent surgery (conservative or radical) and with reported outcomes was calculated. For PIPTB this is 50% (n=6) for conservative surgery and 22.2% (n=2) for radical surgery. Utilising the same criteria but for SIPTB, then the chance of failure for conservative surgery comes to 40% (n=2), while 43.8% (n=7) for radical surgery. Salvage was possible and often successful with surgery alone for IPTB, while for SIPTB salvage was less successful despite the addition of adjuvant treatment. The non-significant difference in treatment outcomes between PIPTB and SIPTB must be interpreted with care as, in general, PIPTB represented less extensive disease and SIPTB was treated more radically and with adjuvant therapy. In addition, the small numbers, heterogeneity in primary and adjuvant treatments and limited follow-up preclude any definitive conclusions being drawn. Furthermore, the traditional approach to sinonasal IP is complete surgical resection, which is difficult to achieve in IPTB due to anatomic factors. Complete resection of IPTB offers the best chance of cure, and this is supported by the literature (37,46), however the optimal way to achieve this has yet to be defined. Any surgery needs to be tailored to both patient and disease factors. Patient factors include age, patient preference and fitness for surgery. Disease factors include PIPTB versus SIPTB, dysplastic or malignant histopathology, and locoregional extent, while the effect of HPV status is unknown as a disease modifying factor. Radical surgery such as temporal bone resection is feasible for IPTB but the associated morbidity may not be justified when there is no evidence of malignant transformation and in situations where a more conservative approach would be curative and less morbid (13,46).
Overall, in the literature IPTB was treated with debulking surgery in six cases. Both patients presented in this report underwent debulking surgery, in part due to patient preference and in part as the chance of surgical cure was low and entailed significant risk. For Case 1, this has provided a degree of symptom control for close to 6 years. For Case 2, surgery was primarily performed to diagnose malignant transformation due to disease progression seen clinically and on imaging. Surgical resection of IPTB especially with involvement of the Eustachian tube is not without significant risk, as shown by Mitchell et al. [2012] where curative resection for SIPTB was achieved but involved utilising a combined transnasal/transtemporal approach and preparing for a carotid injury (52). Debulking surgery, although non-curative, may still play a role in terms of symptom control and providing tissue to aid in diagnosis in carefully selected cases that are not amenable to curative resection, or due to patient and disease factors.
Adjuvant therapy should be considered on a case by case basis and is generally reserved for dysplastic, malignant or recurrent disease, or in cases where the patient is not fit for, or refuses, surgery. HPV vaccination did not seem to dramatically alter the natural history of the disease in the two cases presented, despite its effectiveness being reported in one case of sinonasal IP (10). In the two cases reported on, one was p16 positive and the other negative, while both had advanced disease with only a limited period of observation.
The distinction between PIPTB and SIPTB seems valid based on the current literature limited regarding IPTB, due to the difference in clinicopathologic features, treatment approaches, and outcomes. Risk of bias for the studies were assessed, using a standardised tool developed for observational studies of interventions (9). However, due to the included trials being case reports or series, bias could not be assessed beyond the domains of follow-up and reporting where it was at a high risk for all studies. The limitations of this review include the heterogeneous types of surgery performed; conservative versus radical and variation within these groups; and the inconsistent addition of adjuvant treatments and limited follow-up presented. Most of papers contain only a short duration of follow-up and this would bias towards an under reporting of treatment failures, and in addition the rarity of the disease would likely produce a publication bias against cases with poor outcomes and untreatable disease.
Conclusions
IPTB is poorly understood, and the literature suggests that dividing it into PIPTB and SIPTB may be worthwhile to aid in management. However, whether or not PIPTB and SIPTB are distinct entities remains to be proven. Based upon the limited available evidence the management of IPTB should be complete surgical extirpation, but in certain situations limited surgery such as debulking procedures may offer diagnostic or symptomatic benefit. Adjuvant therapy should primarily be considered in the setting of malignant transformation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/ajo-20-74
Peer Review File: Available at http://dx.doi.org/10.21037/ajo-20-74
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-20-74). RB served as an unpaid editorial board member of the Australian Journal of Otolaryngology from Jan 2019 to Dec 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barnes L. Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol 2002;15:279-97. [Crossref] [PubMed]
- Carlson ML, Sweeney AD, Modest MC, et al. Inverting papilloma of the temporal bone: report of four new cases and systematic review of the literature. Laryngoscope 2015;125:2576-83. [Crossref] [PubMed]
- Stone DM, Berktold RE, Ranganathan C, et al. Inverted papilloma of the middle ear and mastoid. Otolaryngol Head Neck Surg 1987;97:416-8. [Crossref] [PubMed]
- Wenig BM. Schneiderian-type mucosal papillomas of the middle ear and mastoid. Ann Otol Rhinol Laryngol 1996;105:226-33. [Crossref] [PubMed]
- Goudakos JK, Blioskas S, Nikolaou A, et al. Endoscopic resection of sinonasal inverted papilloma: systematic review and meta-analysis. Am J Rhinol Allergy 2018;32:167-74. [Crossref] [PubMed]
- Busquets JM, Hwang PH. Endoscopic resection of sinonasal inverted papilloma: a meta-analysis. Otolaryngol Head Neck Surg 2006;134:476-82. [Crossref] [PubMed]
- Wassef SN, Batra PS, Barnett S. Skull base inverted papilloma: a comprehensive review. ISRN Surg 2012;2012:175903 [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol 2012;65:163-78. [Crossref] [PubMed]
- Sirera G, Videla S, Verges J, et al. Aggressive human papillomavirus (HPV)-11-related sinonasal inverted papilloma in an HIV-infected patient and the quadrivalent HPV vaccine: a case report. AIDS 2015;29:2366-8. [Crossref] [PubMed]
- Santos Torres SM, Castro TW, Bento RF, et al. Middle ear papilloma. Braz J Otorhinolaryngol 2007;73:431. [Crossref] [PubMed]
- Roberts WH, Dinges DL, Hanly MG. Inverted papilloma of the middle ear. Ann Otol Rhinol Laryngol 1993;102:890-2. [Crossref] [PubMed]
- Chhetri DK, Gajjar NA, Bhuta S, et al. Pathology forum. Quiz case 2. Schneiderian-type papilloma of the middle ear. Arch Otolaryngol Head Neck Surg 2001;127:79-80-2. [PubMed]
- de Filippis C, Marioni G, Tregnaghi A, et al. Primary inverted papilloma of the middle ear and mastoid. Otol Neurotol 2002;23:555-9. [Crossref] [PubMed]
- Marioni G, Altavilla G, Busatto G, et al. Detection of human papillomavirus in temporal bone inverted papilloma by polymerase chain reaction. Acta Otolaryngol 2003;123:367-71. [Crossref] [PubMed]
- Blandamura S, Marioni G, de Filippis C, et al. Temporal bone and sinonasal inverted papilloma: the same pathological entity? Arch Otolaryngol Head Neck Surg 2003;129:553-6. [Crossref] [PubMed]
- Cahali S, da Silva FB, Machado MC, et al. Middle ear squamous papilloma: report of a case and literature review. Braz J Otorhinolaryngol 2005;71:396-8. [Crossref] [PubMed]
- Ali RB, Amin M, Hone S. Tinnitus as an unusual presentation of Schneiderian papillomatosis. Ir J Med Sci 2011;180:597-9. [Crossref] [PubMed]
- van der Putten L, Bloemena E, Merkus P, et al. Schneiderian papilloma of the temporal bone. BMJ Case Rep 2013;2013:bcr2013201219 [Crossref] [PubMed]
- Coca-Pelaz A, Gomez-Martinez J, Vivanco-Allende B, et al. Primary inverted papilloma of the middle ear with intracranial invasion. Head Neck 2016;38:E105-7. [Crossref] [PubMed]
- Schaefer N, Chong J, Griffin A, et al. Schneiderian-type papilloma of the middle ear: a review of the literature. Int Surg 2015;100:989-93. [Crossref] [PubMed]
- Mummadi SM, Darr A, Hakim N, et al. A rare case of Schneiderian papilloma of the middle ear presenting with pulsatile tinnitus. Ann R Coll Surg Engl 2018;100:e109-11. [Crossref] [PubMed]
- Eui-Kyung G, Ilyoung C, Se-Joon O, et al. A case of inverted papilloma of the mastoid cavity after cholesteatoma surgery. J Int Adv Otol 2018;14:148-50. [Crossref] [PubMed]
- O'Connell BP, Rivas A, Wanna GB, et al. Inverting papilloma of the middle ear: a case report. Laryngoscope 2010;120:S85. [Crossref]
- Rubin F, Badoual C, Moya-Plana A, et al. Inverted papilloma of the middle ear. Eur Ann Otorhinolaryngol Head Neck Dis 2012;129:207-10. [Crossref] [PubMed]
- Inoue R, Kanazawa T, Morita M, et al. Inverted papilloma of the middle ear. Otol Neurotol 2011;32:e7-8. [Crossref] [PubMed]
- Hasnaoui M, Masmoudi M, Abdeljelil NB, et al. A rare case of primary inverted papilloma of the middle ear. Pan Afr Med J 2019;33:49. [Crossref] [PubMed]
- Adams M, Smith C, Hampton S. Isolated Schneiderian papilloma of the middle ear cleft. BMJ Case Rep 2019;12:e228130 [Crossref] [PubMed]
- Avallone E, Lenarz T, Willenborg K. Case report and review of the literature about the primary Schneider papilloma of the temporal bone with involvement of the skull base. Laryngo-Rhino-Otologie 2019;98:11296.
- Ulkumen B, Kaplan Y, Kıroglu AF, et al. Pediatric inverted papilloma of the middle ear: case report and review of the literature. Int J Pediatr Otorhinolaryngol Extra 2014;9:136-8. [Crossref]
- Ishigaki TY, Kojima H, Moriyama H, et al. A case of inverted papilloma of the middle ear. Oto-Rhino-Laryngology Tokyo 2012;55:235-40. Available online: https://www.embase.com/a/#/search/results?subaction=viewrecord&rid=18&page=2&id=L366100879
- Zhou H, Chen Z, Li H, et al. Primary temporal inverted papilloma with premalignant change. J Laryngol Otol 2011;125:206-9. [Crossref] [PubMed]
- Bayindir E, Hanege FM, Kalcioglu MT, et al. A case of inverted papilloma originating from the middle ear and review of the literature. Case Rep Otolaryngol 2019;2019:3041570 [Crossref] [PubMed]
- Nath J, Das B. Primary inverted papilloma of middle ear and mastoid: a rare case report. J Clin Diagn Res 2016;10:XD01-3. [Crossref] [PubMed]
- Kaddour HS, Woodhead CJ. Transitional papilloma of the middle ear. J Laryngol Otol 1992;106:628-9. [Crossref] [PubMed]
- Vural E, Suen JY, Hanna E. Intracranial extension of inverted papilloma: an unusual and potentially fatal complication. Head Neck 1999;21:703-6. [Crossref] [PubMed]
- Barbosa JL, Pinheiro SD, Freitas MR, et al. Sinonasal inverted papilloma involving the middle ear and the mastoid. Braz J Otorhinolaryngol 2012;78:122. [Crossref] [PubMed]
- Pla-Gil I, Morant Ventura A, Redondo Martínez J, et al. Inverted papilloma of middle ear and temporal bone. Acta Otorrinolaringol Esp (Engl Ed) 2018;69:48-50. [Crossref] [PubMed]
- Trupthi MC, John M, Subbaraj R, et al. Multicentric Inverted Papilloma of Sinonasal Region and the Temporal Bone. Int J Otorhinolaryngol Clin 2018;10:35-8. [Crossref]
- Trahan JRM. Inverting papilloma involving bilateral temporal bones with intracranial extension: case report. Otolaryngology - Head and Neck Surgery 2018;159:272.
- Gomez ED, Yu JL, Brant J. Unusual case of inverted papilloma in the middle ear. Otolaryngology - Head and Neck Surgery 2017;157:262-3.
- Pou AM, Vrabec JT. Inverting papilloma of the temporal bone. Laryngoscope 2002;112:140-2. [Crossref] [PubMed]
- Seshul MJ, Eby TL, Crowe DR, et al. Nasal inverted papilloma with involvement of middle ear and mastoid. Arch Otolaryngol Head Neck Surg 1995;121:1045-8. [Crossref] [PubMed]
- Jones ME, Wackym PA, Said-Al-Naief N, et al. Clinical and molecular pathology of aggressive Schneiderian papilloma involving the temporal bone. Head Neck 1998;20:83-8. [Crossref] [PubMed]
- Mazlina S, Shiraz MA, Hazim MY, et al. Sinonasal inverted papilloma with malignant transformation in the middle ear: a multicentric origin? J Laryngol Otol 2006;120:597-9. [Crossref] [PubMed]
- Acevedo-Henao CM, Talagas M, Marianowski R, et al. Recurrent inverted papilloma with intracranial and temporal fossa involvement: a case report and review of the literature. Cancer Radiother 2010;14:202-5. [Crossref] [PubMed]
- Dingle I, Stachiw N, Bartlett A, et al. Bilateral inverted papilloma of the middle ear with intracranial involvement and malignant transformation: first reported case. Laryngoscope 2012;122:1615-9. [Crossref] [PubMed]
- Ramey SJ, Russo JK, Condrey JM 3rd, et al. Synchronous bilateral inverted papilloma of the temporal bone: case report and review of the literature. Head Neck 2013;35:E240-5. [Crossref] [PubMed]
- Liu ZW, Walden A, Lee CA. Sinonasal inverted papilloma involving the temporal bone via the eustachian tube: case report. J Laryngol Otol 2013;127:318-20. [Crossref] [PubMed]
- Brown CS, Abi Hachem R, Pendse A, et al. Low-grade papillary Schneiderian carcinoma of the sinonasal cavity and temporal bone. Ann Otol Rhinol Laryngol 2018;127:974-7. [Crossref] [PubMed]
- Shen J, Baik F, Mafee MF, et al. Inverting papilloma of the temporal bone: case report and meta-analysis of risk factors. Otol Neurotol 2011;32:1124-33. [Crossref] [PubMed]
- Mitchell CA, Ebert CS, Buchman CA, et al. Combined transnasal/transtemporal management of the eustachian tube for middle ear inverted papilloma. Laryngoscope 2012;122:1674-8. [Crossref] [PubMed]
- Kainuma K, Kitoh R, Kenji S, et al. Inverted papilloma of the middle ear: a case report and review of the literature. Acta Otolaryngol 2011;131:216-20. [Crossref] [PubMed]
- Barry JY, Le CH, Khan R, et al. Multifocal inverting papilloma of the sinonasal cavity and temporal bone. Otolaryngology Case Reports 2017;2:33-6. [Crossref]
- Haywood EB, Fuller C, Hill GW 3rd, et al. Multifocal sinonasal inverted papilloma with middle ear involvement. Proc (Bayl Univ Med Cent) 2017;30:457-8. [Crossref] [PubMed]
- Scheuer VB, A, Schorn B, Schick B. An inverted papilloma of the middle ear. Laryngo-Rhino-Otologie 2018;97:S15-6.
- Bold EL, Wanamaker JR, Hughes GB, et al. Adenomatous lesions of the temporal bone immunohistochemical analysis and theories of histogenesis. Am J Otol 1995;16:146-52. [PubMed]
- Altug T, Sunar O, Bilgin H. Inverted papilloma. Apropos of a multicentric case. Rev Laryngol Otol Rhinol (Bord) 1989;110:299-301. [PubMed]
- Suh JD, Chiu AG. What are the surveillance recommendations following resection of sinonasal inverted papilloma? Laryngoscope 2014;124:1981-2. [Crossref] [PubMed]
- Frasson G, Cesaro S, Cazzador D, et al. High prevalence of human papillomavirus infection in sinonasal inverted papilloma: a single-institution cohort of patients. Int Forum Allergy Rhinol 2020;10:629-35. [Crossref] [PubMed]
- Lawson W, Schlecht NF, Brandwein-Gensler M. The role of the human papillomavirus in the pathogenesis of Schneiderian inverted papillomas: an analytic overview of the evidence. Head Neck Pathol 2008;2:49-59. [Crossref] [PubMed]
- Zhao RW, Guo ZQ, Zhang RX. Human papillomavirus infection and the malignant transformation of sinonasal inverted papilloma: a meta-analysis. J Clin Virol 2016;79:36-43. [Crossref] [PubMed]
- Mohajeri S, Lai C, Purgina B, et al. Human papillomavirus: an unlikely etiologic factor in sinonasal inverted papilloma. Laryngoscope 2018;128:2443-7. [Crossref] [PubMed]
- Attlmayr B, Derbyshire SG, Kasbekar AV, et al. Management of inverted papilloma J Laryngol Otol 2017;131:284-9. review. [Crossref] [PubMed]
Cite this article as: Mclean T, Dhillon K, Lyons B, Briggs R. Inverted papilloma of the temporal bone: recent experience and a systematic review of management. Aust J Otolaryngol 2021;4:23.


