
Dysphagia in laryngomalacia: a prospective cohort study
Introduction
Laryngomalacia was first recognised in 1853, described by Thomson in 1892, and named by Hollinger in 1960 (1). Most infants grow out of the disease. A total of 5–30% require surgical intervention due to airway obstruction with desaturation and or apnea, failure to thrive (FTT) with the increased work of breathing, or dysphagia due to difficulties with the suck-swallow-breathe coordination (2-7). The symptoms usually occur in the first two weeks of life, peak around 6 months prior to spontaneous resolution by 12 to 24 months (4,5,8).
Irace et al. reported 14.9% rate of silent aspiration in their patients with laryngomalacia (8). The disruption of the suck-swallow-breathe sequence and airway protection is thought to result in dysphagia in laryngomalacia (8). This may be manifested as coughing, choking, gagging, regurgitation, emesis, slow and or inefficient feeding (8-10). In total, 14–88% of patients with laryngomalacia has dysphagia (9,11,12). Of those needing supraglottoplasty, up to 28% develop new dysphagia (7,12-14). Currently, there are no studies looking prospectively at the incidence of dysphagia in both the surgical and conservative groups, whether it worsens following surgical intervention and if dysphagia resolves in both of these groups. The aim of our prospective cohort study is to describe the incidence of dysphagia in patients with laryngomalacia, the effect of interventions on this, and the period it persists in these infants, with and without surgical intervention. The author presents the following article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-21-44/rc).
Methods
We obtained the institutional review board approval (IRB No. 32573) for the prospective data collection from August 2017 to May 2018 of consecutive cohort of patients with laryngomalacia referred to the paediatric otolaryngology service seen by the author at the only tertiary paediatric centre in Western Australia. The study period ended in January 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained.
Baseline assessments
We collected patient demographics, risk factors for dysphagia, swallow function, and management based on a purposefully designed baseline clinic questionnaire.
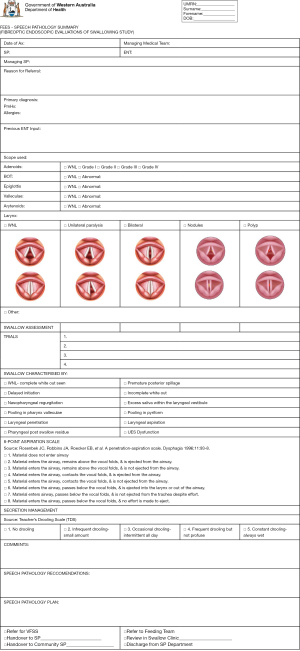
All patients were evaluated with a flexible nasendosopy (FNE) to confirm the diagnosis of laryngomalacia. The severity is classified as mild, moderate and severe according to Thompson’s severity scoring system (15). Their swallowing function was assessed by a single paediatric otolaryngologist in conjunction with a paediatric speech language pathologist (SLP) with functional endoscopic evaluation of swallowing (FEES). A baseline FEES form was filled (Figure 1). Endoscopic findings of laryngopharyngeal/gastroesophageal reflux disease (LPR/GORD) is defined as arytenoids edema and erythema, postcricoid edema/pachydermia, or vocal fold edema (16). If there were any evidence of penetration or aspiration, diet modification was instituted starting at level 1 or 2 (17), or adjunctive changes to the bottle and teat. If patients did not tolerate FEES, then videofluoroscopic swallowing study (VFSS) was organised.
All patients underwent a trial of reflux therapy with proton pump inhibitor (PPI), at a dose of 1 mg/kg/day. Adjunctive counselling was provided by the SLP for standard reflux precaution like prevention of over-feeding, or elevation at the time of feeding.
The period of follow up of both conservative and post-operative patients was determined by the severity of their symptoms. They were all followed up in the outpatient setting at the swallow clinic, as well as by the managing SLP in between swallow clinic appointments, either in person or telephonically.
Surgery
The indication for supraglottoplasty included respiratory issues, work of breathing resulting in FTT, and sleep apnea.
Those that required surgical intervention underwent spontaneous ventilation, with a stat dose of intravenous dexamethasone (0.15 mg/kg) and topical lignocaine applied to the vocal fold at (0.1 mg/kg) given by the anaesthesiologist. After application of topical adrenaline neuropatties, the cool steel technique was used, with micro-scissor divisions of the tight aryepiglottic folds, followed by excisions of the redundant arytenoid mucosa, preserving the inter-arytenoid space to prevent supraglottic stenosis or posterior webbing.
Patients were admitted overnight and those under three to 6 months of age, and or significant medical co-morbidities were admitted to the paediatric intensive care unit (PICU) for monitoring per the department’s protocol.
Post-operative assessments
All the patients were assessment by the SLP. Post-operative FEES was performed in the immediate post-operative period if there were any concerns regarding their feeding. All the patients were placed on PPI in the post-operative period until review in clinic, at 6 weeks post-operatively, where the post-operative FEES was performed based on clinical need.
Statistical analysis
Data was analysed with SPSS Statistics (SPSS, Inc., an IBM Company, Chicago, IL, USA) software. Independent sample Student t-test compared the mean between groups; paired sample Student t-tests compared means of variables. Statistical significance was defined as 5% (α=0.05).
Results
Patients demographics
There were 20 patients, with a mean age at diagnosis was 2.28 (range, 0.2–6.5) months. The mean gestational age was 38.7 (range, 35–42) weeks. The gender was equally distributed at 10 in each group.
Pregnancy issues were reported in 8 patients: hypertension (n=1, 5%), hyperemesis (n=1), placental abruption (n=2, 10%), and polyhydromnios with gestational diabetes (n=2). Delivery issues were recorded in half of the patients: nuchal cord (n=2, 10%), labour induced for lack of progress (n=1, 5%), emergency caesarean section (CS) for fetal distress (n=4, 20%), placental abruption (n=2), and premature rupture of membrane with forceps delivery (n=1).
Five babies had postnatal issues: aspiration (n=1, 5%), desaturation with feeds (n=1), neonatal jaundice (n=1) and requirement for resuscitation (n=2, 10%). None of these patients had respiratory, or history of intubation. One baby had patent ductus arteriosus (PDA), 1 had neurofibromatosis type 1.
Laryngomalacia characteristics
The primary presentation was stridor (n=13, 65%), followed by both stridor and dysphagia (n=5, 25%), and dysphagia alone (n=2, 10%). Four (20%) reported observed cyanosis, 5 (25%) had observed apnea, 9 (45%) reported moist respiratory sounds during/after feeds, 8 (40%) had FTT, 1 (5%) had an acute life-threatening event admission, and two (10%) had obstructive sleep apnea (OSA).
Nine patients (45%) had mild laryngomalacia, 4 (20%) moderate and 7 (35%) had severe laryngomalacia.
While 7 had a history of regurgitation or emesis, 19 (95%) had clinical evidence of LPR/GORD observed during the scope. All the patients were treated with PPI.
Two patients had nasogastric tube (NGT) placed due to FTT. One had it inserted for 25 days. The other had it for 451 days due to severe vomiting and GORD. No patients had gastrostomy tube.
Supraglottoplasty patients
Supraglottoplasty patients: patient demographics
There were 4 females (44.4%) and 5 (55.6%) males. The mean diagnosis age was 1.69 (range, 0.2–3.5) months. The most common concomitant airway diagnosis found are deep interarytenoid notch (n=2), tracheomalacia (n=2) and laryngeal cleft (n=1). They had no significant medical co-morbidities.
Majority of the surgical patients presented with stridor (n=4, 44.4%), 2 (22.2%) presented with dysphagia, while 3 (33.3%) presented with both. Laryngomalacia severity was mild in 1 (11.1%), moderate in 2 (22.2%), and severe in most (n=6, 66.7%).
All the supraglottoplasty were performed via the cold steel technique. The mean surgical age was 2.6 (range, 0.20–4.5) months. The main indication for surgery were airway issues. Of those with additional indications, 5 (55.56%) were for FTT and 1 (11.11%) for dysphagia. The mean length of stay (LOS) was 3.89 (range, 1–11) days. Patient 8 had a prolonged LOS (11 days) due to dysphagia.
Supraglottoplasty patients: pre-operative swallow
All but one had FEES performed prior to surgery, due to the need for surgery emergently. A third of these patients had a normal pre-operative FEES. The FEES findings are summarised in Table 1.
Table 1
| Patients | Initial FEES findings | Initial diet modification | 1st repeat FEES findings | VFSS findings | Final diet modification | Oral aversion | Dysphagic days |
|---|---|---|---|---|---|---|---|
| Patient 1 | 0 | 0 | NA | NA | 0 | No | 0 |
| Patient 2 | 3 | 0 | NA | NA | 0 | No | 267 |
| Patient 3 | 1 | 0 | NA | NA | 0 | No | 98 |
| Patient 4 (SG) | 2, 5 | 0 | 1 (day 1 post op) | NA | 2 | Yes | 666 |
| Patient 5 | 1, 3, 5 | 0 | NA | NA | 0 | No | 0 |
| Patient 6 (SG) | 0 | 0 | 1 (day 22 post op) | NA | 2 | No | 72 |
| Patient 7 | 2–5 | 2 | NA | NA | 1 | No | 395 |
| Patient 8 (SG) | Not performed | 0 | 1 (day 42 post op) | 8 (day 105 post op) | 0 | Yes | 306 |
| Patient 9 | 2 | 0 | NA | NA | 0 | No | 0 |
| Patient 10 | 1, 3, 5 | 1 | NA | NA | 1 | No | 155 |
| Patient 11 | 3, 5 | 1 | NA | NA | 1 | No | 136 |
| Patient 12 (SG) | 5 | 2 | 2 (day 145 post op) | 2 | No | 521 | |
| Patient 13 | 1 | 0 | NA | NA | 0 | No | 0 |
| Patient 14 (SG) | 1–3, 5 | 0 | 1 (day 82 post op) | 1 (day 96 post op) | 2 | Yes | 727* |
| Patient 15 | 1, 3, 5 | 1 | NA | NA | 1 | No | 69 |
| Patient 16 (SG) | 5 | 1 | 1 (day 77 post op) | 1 (day 5 post op) | 2 | No | 77 |
| Patient 17 (SG) | 6 | 1 | 1 (day 32 post op) | NA | 1 | No | 72 |
| Patient 18 (SG) | 0 | 0 | NA | NA | 0 | No | 0 |
| Patient 19 | 0 | 0 | NA | NA | 0 | No | 0 |
| Patient 20 (SG) | 1, 5 | 0 | 6 (day 1 post op) | 1 (day 112 post op) | 0 | No | 484 |
*, ongoing at the end of study. FEES findings: 0, normal; 1, poor secretion management (secretions in base of tongue/valleculae/pyriform fossa pre swallow); 2, premature posterior spillage; 3, feed pooling in valleculae/pyriform fossa; 4, aspiration; 5, penetration. VFSS findings: 0, normal; 1, mild oral dysphagia; 2, mod oral dysphagia; 3, severe oral dysphagia; 4, mild pharyngeal dysphagia; 5, mod pharyngeal dysphagia; 6, severe pharyngeal dysphagia; 7, penetration; 8, aspiration. FEES, functional endoscopic evaluation of swallowing; VFSS, videofluoroscopic swallowing study; SG, supraglottoplasty; NA, not available; post op, post-operative.
Supraglottoplasty patients: post-operative swallow
The post-operative FEES was done on median day 37 (range, 1–145 days). Only 1 (11.11%) who had normal FEES pre-operatively remained normal. The majority of the patients (n=6, 66.67%) had poor secretions management, 1 had premature posterior spillage, and another penetration (Table 1).
In the immediate post-operative period, diet modification was instituted in 7 (77.78%) patients: 1 level 1 and 6 (30%) level 2 (Table 1). All had resolved their respiratory symptoms. They all eventually returned to normal diet but the median dysphagia time was 191.5 (range, 0–666) days (Table 1). Oral aversion developed in a third of these patients (patients 4, 8 and 14) (Table 1).
VFSS was performed in 4 (20%) patients post-operatively due to their persistent dysphagia on median day 104 (range, 5–306 days): 3 (15%) had mild oral dysphagia, and 1 had silent aspiration (Table 1). Unfortunately, this was the only patient who did not have pre-operative FEES.
Conservative patients
Conservative patients: patients demographics
There were 6 females (54.5%) and 5 (45.5%) males. The mean diagnosis age was 2.76 (range, 0.5–6.5) months. Only one patient had cardiac issue, and another NF-1.
Majority presented with stridor (n=9, 81.8%), while 2 (18.2%) presented with concomitant dysphagia. Laryngomalacia severity was mild in most (n=8, 72.7%), moderate in 2 (18.2%), and severe in 1 (9.1%).
Conservative patients: dysphagia findings
All of the patients had FEES performed. These were normal in 4 (36.4%). The clinical findings are shown in Table 1.
Diet modification was instituted in 4 (36.36%) patients: 3 level 1 and 1 level 2 (Table 1). At the end of the study period, all the dysphagia had resolved. The patient with NF-1 remained dysphagic for the longest period (395 days). The median dysphagia time for this population was 69 (range, 0–395) days.
Dysphagia
For the entire patient population, 6 (30%) had normal swallow. The median dysphagia period was 77 (range, 0–666) days (Table 1).
Increasing severity of laryngomalacia was associated with FTT (P=0.00), emesis (P=0.02), and longer period of dysphagia [P=0.00; confidence interval (CI): −271.74 to −73.7].
Fifteen (75%) patients had signs and symptoms of dysphagia. Of all the clinical signs of dysphagia, choking was most commonly reported (Table 2). Six patients reported two or more concomitant signs.
Table 2
| Clinical signs & symptoms of dysphagia | Total frequency (%) | Conservative group | Surgical group |
|---|---|---|---|
| None | 5 [25] | 4 | 1 |
| Choking | 9 [45] | 4 | 5 |
| Moist respiratory sounds post feeds | 10 [50] | 3 | 3 |
| Coughing | 4 [20] | 2 | 2 |
| Emesis | 6 [30] | 3 | 4 |
| Regurgitation | 4 [20] | 1 | 3 |
| Slow feeding | 1 [5] | 0 | 1 |
Some patients may have more than one clinical finding of FEES. FEES, functional endoscopic evaluation of swallowing.
The correlations of pre-operative symptoms and prolonged period of dysphagia include cyanosis (P=0.03; CI: −459.60 to −20.14), cough with feeds (P=0.04; CI: −508.6 to −13.19), moist respiratory sounds post feeds (P=0.04; CI: −368.33 to −4.21), regurgitation (P=0.00; CI: −470.5 to −112.44), emesis (P=0.00; CI: −470.5 to −112.44), FTT (P=0.04; CI: −474.13 to −20.58), per-operative NGT (P=0.01; CI: −893.78 to −143.55), FEES findings of penetration (P=0.047; CI: −389.81 to −3.41).
Dysphagia: clinical signs and symptoms vs. FEES
Generally clinical signs and symptoms correlated with abnormal FEES findings, except two who had normal FEES despite choking and moist respiratory sounds post feeds; and three who was asymptomatic but had abnormal FEES findings (poor secretion management, premature spillage, and another feed pooling with penetration).
Clinical signs and symptoms choking and or cough is consistent with FEES findings of penetration and or aspiration in 14 patients. Two patients were asymptomatic despite FEES findings of penetration and or aspiration. Four were false positive. Clinical signs and symptoms are not a reliable method (P=0.06) to predict FEES findings of penetration and or aspiration.
Unsurprisingly, pre-operative diet modification increases the risk of post-operative diet modification [odds ratio (OR): 9.6; CI: 0.88 to 105.17; P=0.04].
Dysphagia & oral aversion
The pre-operative signs and symptoms associated with an increased risk of oral aversion include cyanosis (OR: 15; CI: 0.9 to 251.1; P=0.03), regurgitation (OR: 15; CI: 0.9 to 251.1; P=0.03), slow feeds (OR: 0.11; CI: 0.03 to 0.40; P=0.02), FTT (OR: 1.6; CI: 0.9 to 2.7; P=0.02) and premature spillage on pre-operative FEES (OR: 15; CI: 0.90 to 251.01; P=0.03).
All the patients with oral aversion were in the surgical group (OR: 1.5; CI: 0.95 to 2.38; P=0.04). It is associated with a longer period of dysphagia (486 vs. 138 days; P=0.02; CI: −630.98 to −65.02).
Dysphagia: follow up period
The median follow up time was 209 (range, 49–727) days. Pre-operative predictors of longer follow up include patients with pre-operative NGT (P=0.01; CI: −651.48 to −254.08), cough (P=0.01; CI: −445.78 to −79.22), regurgitation (P=0.00; CI: −495.18 to −216.07), emesis (P=0.03; CI: −480.79 to −44.92), slow feeds (P=0.01; CI: −812.17 to −133.1), pre-operative findings of premature spillage on FEES (P=0.01; CI: −449.39 to −86.86), pre-operative findings of penetration on FEES (P=0.01; CI: −360.06 to −54.34), and oral aversion (P=0.00; CI: −510.81 to −113.51).
Discussion
Pre-operative dysphagia
Dysphagia as a primary presentation of laryngomalacia has been reported to occur in 0.5–11% (3,18). Respiratory symptoms, recurrent chest infections or pneumonias have been found to be strong predictors of dysphagia (19). The consequences of dysphagia is increased risk of aspiration-induced chronic lung disease, malnutrition, neurodevelopmental problems and stressful interactions with their caregivers (20). Dysphagia and aspiration may be under reported as children often have silent aspiration, and the lack of overt symptoms or clinical signs may result in chronic airway disease if untreated (8,20-22). Liquids were more commonly aspirated during swallow, purees after and solids during and before swallows (21). Durvasula noted that post-operative dysphagia was higher in preterm (32.5%) compared to term (6.6%) infants (23). Severe dysphagia with slower improvement is associated with prematurity when gestation age is less than 32 weeks (23). The rate of aspiration on VFSS post-operatively is 68% in term and 54% preterm until 1 year, 52% vs. 57% until 18 months, 16.7% vs. 33% after 18 months (23).
In Chun et al.’s retrospective study, patients were assessed with clinical evaluation of swallowing if there was concerns for post-operative feeding difficulty or to provide clearance for safe feeding (2). While only three patients showed signs of coughing or choking with oral feeds, all their patients on VFSS demonstrated aspiration (2). Irace et al. evaluated all patients with laryngomalacia for dysphagia with recurrent respiratory issues, feeding difficulty or both, with VFSS (8). A total of 90.1% had swallowing dysfunction, while 70.4% had penetration or aspiration (8). A total of 42.3% had aspiration and 98.3% had silent aspiration (8). They acknowledged that they may underestimate the prevalence of the patients not formally evaluated for silent aspiration. Arvedson et al. reported 94% rate of silent aspiration in their patients with multiple disabilities (21). Pre-operative dysphagia is noted in 72.5% preterm infants and 58.5% term infants in Durvasula et al.’s review (23). VFSS performed in all preterm infants were all abnormal pre-operatively (23). Other studies reported 50.3–88% rate of dysphagia in children with laryngomalacia (9,18,19). Laryngeal penetration and aspiration are common in children with laryngomalacia (8). Svystun et al. reported 70% of their patient population choked or coughed on liquids, 20% had a history of prolonged feeding and 26% had vomiting (19).
In our study, all but two (35 weeks) of the patients were term. Only one had cardiac issue (PDA) and another syndromic (NF-1). Seventy-five percent had signs and symptoms of dysphagia. All of these patients were term infants (≥37 weeks gestation). FEES was abnormal in 3 (13.6%) patients who were reported to have normal swallow. As in previous studies, clinical signs and symptoms were found to be unreliable to predict penetration and aspiration. FESS was abnormal in 73% of the patients in the conservative group; 75% in the surgical group. The clinical findings of our study is comparable with Svystun et al.’s: 45% reported choking, 20% coughing, 5% had prolonged feeding, and 35% had emesis.
Post-operative dysphagia
While airway symptoms improve quickly, dysphagia and aspiration improve slowly, gradually and can be variable (23). The effect of supraglottoplasty on dysphagia has been reported to be variable (24). Supraglottoplasty reduces anatomical obstruction, improves laryngeal tone and may improve laryngeal sensation (23). In the post-operative setting, the physiology alterations of the timing and coordination of sucking, swallowing and breathing secondary to the laryngeal anatomy alteration may result in transient dysphagia (2). The reduction in the volume of the obstructive tissue may change the spatial relationship to affect the sensorimotor function until the child adapts (2). Richter et al. postulated that supraglottoplasty exposes neural endings in the densest area of superior laryngeal nerve fibers over the aryepiglottic folds and supra-arytenoid tissue, enhancing laryngeal sensation and airway protection (12). This with the natural laryngeal maturation may reduce aspiration. Wertz et al.’s study reported a median dysphagic days of 165 days (18). However, these improve irrespective of gestational age, with majority by 18–24 months (23).
In total, 3–25% of patients have been reported to have a transient dysphagia (2,7,13,18). Richter et al. (12) reported 88% of penetration and 72% of aspiration on FEES in their patients with severe laryngomalacia who underwent supraglottoplasty. In total, 82% of patients with penetration and 86% with aspiration had resolution post-operatively. In Durvasula et al.’s study, VFSS performed in all preterm infants were all abnormal pre-operatively and half of these resolved on VFSS post-operatively; whilst in 88.89% term infants with a 61.1% improvement (23).
In our study, the median dysphagic days for our entire study population was 77 days. This is 2.5 times longer in the supraglottoplasty group (191.5 days) compared to those in the conservative group (69 days). This is consistent with Wertz et al.’s study (median 165 days). Our follow up ranged from 49 to 727 days.
In total, 78% of our patients had diet restrictions as many (75%) of them were identified to have dysphagia pre-operatively. All our patients eventually resolved their dysphagia (median follow up of 209 days; range, 49–727 days). The predictors of prolonged dysphagia were those with more severe laryngomalacia and had cyanosis, struggled with feeds (cough, moist respiratory noises, regurgitation, emesis, FTT, pre-operative NGT, oral aversion) and pre-operative penetration on FEES. This is consistent with the findings of Wertz et al., with a higher risk of dysphagia in those with pre-operative diet modification, pre-operative NGT, pre-operative swallowing dysfunction and oral aversion (18). Patients with more severe disease are more likely to be dysphagic (9).
Limitations
This is the first study to report objective swallowing assessment in patients pre- and post-operatively. However, the conclusions of our study need to be drawn with caution due to several limitation. The main limitation of this study is the small sample size and it is a single surgeon’s patient cohort. Other causes for persistent post-operative dysphagia includes prematurity, GORD, cardiac disease, genetic or congenital anomalies (8,23). However due to the small sample size, we are not able to draw meaningful conclusions regarding these risk factors.
Patients are selected into the conservative group due to the milder laryngomalacia compared with the surgical group. It is therefore not surprising they had milder dysphagia on FEES, and it tends to improve quicker.
Whilst all but one patient had pre-operative FEES, post-operative FEES and VFSS was performed based on clinical needs, in only 8 patients. A more uniform repeat FESS for the conservative group, post-operative FEES and or VFSS may pick up less severe dysphagia. There is no validated scoring system for paediatric FEES, and the observation remains subjective.
There is no uniform follow up interval since it is determined by both the surgeon and the SLP, depending on the severity of their symptoms, until the dysphagia has completely resolved.
Implications
This prospective cohort study has demonstrated that dysphagia is present in majority of patients with laryngomalacia. FESS and or VFSS should be performed at baseline and regular set intervals to assess the degree of dysphagia, presence of silent aspiration, and document any improvement or deterioration.
Airway symptoms are normally more pronounced and thus easily recognised. However, dysphagia can contribute to FTT and require ongoing management to prevent long term complications like oral aversion or lower respiratory tract issues. Airway symptoms tend to resolve soon after surgery, dysphagia persists for longer in both groups of patients.
Patients in the surgical group are at risk of developing oral aversion due to the degree of dysphagia, which is an important morbidity that is often not recognised by clinicians. The patient’s family should be counselled regarding dysphagia, potential complications and its prognosis.
Conclusions
Dysphagia is common in patients with laryngomalacia. Many studies have demonstrated that dysphagia, as well as silent aspiration is often overlooked due to the lack of clinical signs. Objective assessments of swallowing function is important in these patients. Dysphagia lasts longer in patients who had supraglottoplasty, and is an important co-morbidity with important implications on their growth, has the potential to result in oral aversion, as well as respiratory complications. This impacts not only their quality of life but also that of their family.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-21-44/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-21-44/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-21-44/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-21-44/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Child and Adolescents Health Services (No. 32573). Informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Belmont JR, Grundfast K. Congenital laryngeal stridor (laryngomalacia): etiologic factors and associated disorders. Ann Otol Rhinol Laryngol 1984;93:430-7. [Crossref] [PubMed]
- Chun RH, Wittkopf M, Sulman C, et al. Transient swallowing dysfunction in typically developing children following supraglottoplasty for laryngomalacia. Int J Pediatr Otorhinolaryngol 2014;78:1883-5. [Crossref] [PubMed]
- Cooper T, Benoit M, Erickson B, et al. Primary Presentations of Laryngomalacia. JAMA Otolaryngol Head Neck Surg 2014;140:521-6. [Crossref] [PubMed]
- Dickson JM, Richter GT, Meinzen-Derr J, et al. Secondary airway lesions in infants with laryngomalacia. Ann Otol Rhinol Laryngol 2009;118:37-43. [Crossref] [PubMed]
- Apps JR, Flint JD, Wacogne I. Towards evidence based medicine for paediatricians. Question 1. Does anti-reflux therapy improve symptoms in infants with laryngo-malacia? Arch Dis Child 2012;97:385-7; discussion 387. [Crossref] [PubMed]
- Chandra RK, Gerber ME, Holinger LD. Histological insight into the pathogenesis of severe laryngomalacia. Int J Pediatr Otorhinolaryngol 2001;61:31-8. [Crossref] [PubMed]
- Eustaquio M, Lee EN, Digoy GP. Feeding outcomes in infants after supraglottoplasty. Otolaryngol Head Neck Surg 2011;145:818-22. [Crossref] [PubMed]
- Irace AL, Dombrowski ND, Kawai K, et al. Evaluation of Aspiration in Infants With Laryngomalacia and Recurrent Respiratory and Feeding Difficulties. JAMA Otolaryngol Head Neck Surg 2019;145:146-51. [Crossref] [PubMed]
- Simons JP, Greenberg LL, Mehta DK, et al. Laryngomalacia and swallowing function in children. Laryngoscope 2016;126:478-84. [Crossref] [PubMed]
- Thompson DM. Abnormal sensorimotor integrative function of the larynx in congen-ital laryngomalacia: a new theory of etiology. Laryngoscope 2007;117:1-33. [Crossref] [PubMed]
- Thottam PJ, Simons JP, Choi S, et al. Clinical relevance of quality of life in laryn-gomalacia. Laryngoscope 2016;126:1232-5. [Crossref] [PubMed]
- Richter GT, Wootten CT, Rutter MJ, et al. Impact of supraglottoplasty on aspiration in severe laryngomalacia. Ann Otol Rhinol Laryngol 2009;118:259-66. [Crossref] [PubMed]
- Anderson de Moreno LC, Matt BH. The effects of prematurity on incidence of aspi-ration following supraglottoplasty for laryngomalacia. Laryngoscope 2014;124:777-80. [Crossref] [PubMed]
- Schroeder JW Jr, Thakkar KH, Poznanovic SA, et al. Aspiration following CO(2) laser-assisted supraglottoplasty. Int J Pediatr Otorhinolaryngol 2008;72:985-90. [Crossref] [PubMed]
- Thompson DM. Laryngomalacia: factors that influence disease severity and outcomes of management. Curr Opin Otolaryngol Head Neck Surg 2010;18:564-70. [Crossref] [PubMed]
- May JG, Shah P, Lemonnier L, et al. Systematic review of endoscopic airway findings in children with gastroesophageal reflux disease. Ann Otol Rhinol Laryngol 2011;120:116-22. [Crossref] [PubMed]
- Lam P, Stanschus S, Zaman R, et al. The international dysphagia diet standardisation initiative (IDDSI) framework: The kempen pilot. British Journal of Neuroscience Nursing 2017;13:S18-S26. [Crossref]
- Wertz A, Ha JF, Driver LE, et al. Change in swallow function after supraglottoplasty. Aust J Otolaryngol 2021;4:3. [Crossref]
- Svystun O, Johannsen W, Persad R, et al. Dysphagia in healthy children: Character-istics and management of a consecutive cohort at a tertiary centre. Int J Pediatr Otorhinolaryngol 2017;99:54-9. [Crossref] [PubMed]
- Lefton-Greif MA, Carroll JL, Loughlin GM. Long-term follow-up of oropharyngeal dysphagia in children without apparent risk factors. Pediatr Pulmonol 2006;41:1040-8. [Crossref] [PubMed]
- Arvedson J, Rogers B, Buck G, et al. Silent aspiration prominent in children with dysphagia. Int J Pediatr Otorhinolaryngol 1994;28:173-81. [Crossref] [PubMed]
- Sheikh S, Allen E, Shell R, et al. Chronic aspiration without gastroesophageal reflux as a cause of chronic respiratory symptoms in neurologically normal infants. Chest 2001;120:1190-5. [Crossref] [PubMed]
- Durvasula VS, Lawson BR, Bower CM, et al. Supraglottoplasty in premature infants with laryngomalacia: does gestation age at birth influence outcomes? Otolaryngol Head Neck Surg 2014;150:292-9. [Crossref] [PubMed]
- Kanotra SP, Vaitaitis V, Hopkins H, et al. Impact of supraglottoplasty on parental preception of swallowing using a 10 question swallowing index. Int J Pediatr Otorhinolaryngol 2018;109:122-6. [Crossref] [PubMed]
Cite this article as: Ha JF. Dysphagia in laryngomalacia: a prospective cohort study. Aust J Otolaryngol 2022;5:14.


