
Laryngopharyngectomy surgery in a small volume regional centre—is it safe, feasible, and effective?
Introduction
Head and neck cancer is the fifth most common cancer worldwide (1). More than 90% of these tumours are squamous cell carcinomas (2). Most individuals have a history of exposure to tobacco and alcohol (3).
The primary goal of the treatment is to achieve the highest locoregional control with the least functional disability. Suggested management for advanced hypopharyngeal and laryngeal cancers include organ preservation with primary chemoradiotherapy (CRT), primary laryngopharyngectomy (LP) followed by CRT, or salvage surgery (4). The 5-year survival rate of advanced hypopharyngeal and laryngeal cancer vary from 33% to 48% (5).
Rural Australians are more likely to die within 5 years of a cancer diagnosis than people from metropolitan areas (6). Rural patients tend to present with more advanced disease compared to urban patients. Despite policies and initiatives to reduce barriers in accessing care, rural head and neck cancer patients still commonly travel long distances to access specialist care, often away from their social supports for extended periods (7). The emotional, financial and social impact of this is particularly evident amongst remote Aboriginal Australian patients, where compliance becomes a significant issue (8).
This study reviews our experience in treating advanced hypopharyngeal and laryngeal malignancies across Australia’s Northern Territory, where the population of nearly 300,000 is distributed across an area roughly 1.35 million km2 (approximately six times the size of the United Kingdom). Most patients come from remote regions within the state. These patients previously had to travel interstate to receive their care.
Over the last 15 years, with local expertise and resources development, increased numbers of head and neck cancers have been treated in Royal Darwin Hospital. There is much debate about should receive their cancer care. Should our patients have all their cancer care interstate in larger volume urban centres, or is it just as safe, feasible and effective to treat them locally, where they have better socio-cultural support?
This study aims to demonstrate that laryngopharyngectomy can be just as safe and effective with sufficient local expertise and resources when performed in small volume centres. We present the following article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-21-19/rc).
Methods
A retrospective chart review was performed on all patients with advanced (Stage 3 and 4) hypopharyngeal tumours treated surgically with laryngopharyngectomy between 2009 and 2021 at the Royal Darwin Hospital, Northern Territory, Australia. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee (TEHREC EC 00153). Informed consent was waived.
The demographic analysis included age, gender, indications for laryngopharyngectomy, ethnicity (Aboriginal vs. non-Aboriginal), and location (metropolitan vs. remote) using the Department of Health geographical classification system (9).
The primary outcomes of interest were disease-free survival and complication rates. As a secondary outcome, we also assessed surgical efficiency, using lymph node yield and surgical margins as a surrogate marker. Comparisons were made to evaluate the impact of rurality and Aboriginal status on the outcomes.
Two main contributing factors for complications analyzed were malnourishment risk and salvage surgery. The malnourishment risk was assessed by calculating the nutrition risk index (NRI). It takes into consideration serum albumin, present and usual weight. Depending on their score, patients were categorized into no risk (score >100); mild risk (97.5–100); moderate risk (835–97.5) and severe risk (<83.5) of malnutrition. The term ‘primary laryngectomy’ was used if surgery preceded coadjuvant radiotherapy. The term ‘salvage laryngectomy’ was used when radiotherapy was given before surgery.
Mortality in patients was analyzed as all-cause if the cause of death was not related to the laryngeal of hypopharyngeal cancer and disease-specific if the cause of death was related to a recurrence or metastatic disease (Table 1). Local recurrence was defined as an invasive carcinoma developing six months after the conclusion of the curative treatment. Regional and distal recurrences were defined as the presence of lesions with the same histological type in regional lymph nodes or distant sites, respectively, after the completion of the initial treatment.
Table 1
| Cause of death | Number [%] |
|---|---|
| Stoma site locoregional recurrence | 2 [16] |
| Exacerbation COPD and CCF | 1 [8] |
| General decline, refused all treatment | 1 [8] |
| Hypocalcaemia, sepsis | 1 [8] |
| Malignant bowel obstruction sec to metastatic colorectal cancer | 1 [8] |
| Metastatic mediastinal disease | 1 [8] |
| Pleural effusion, metastatic disease | 3 [28] |
| Non-small cell lung cancer | 1 [8] |
| Sepsis other | 1 [8] |
| Grand total | 12 [100] |
COPD, chronic obstructive pulmonary disease; CCF, congestive cardiac failure.
Statistical analysis
For statistical analysis, a Paired sample t-test and χ2 analyses were performed to determine any statistically significant differences between the patient groups. Survival curves were created using the Kaplan-Meier method, and significant differences among the actuarial curves were tested by Log-rank test. The survival analysis results were described as a 5-year mean survival in percentage and a mean survival time in years. The statistical analysis was performed using the Software for Statistics and Data Science (STATA) for Windows StataCorp version 16.0. Values of P<0.05 were considered statistically significant.
Results
Demographics
Thirty-five patients were identified and included in our chart review. All patients were discussed in the multidisciplinary head and neck tumour board before undergoing laryngopharyngectomy for advanced hypopharyngeal or laryngeal cancer.
The patients were predominantly male (94%), with age at diagnosis ranging from 44 to 80 years old, with a mean age of 61.8 (SD 8.7, 58.9–64.6 years). Forty-eight per cent of the population (n=17) lived in rural regions at the time of the diagnosis, while 52% (n=18) lived in metropolitan areas. Of the patients living in rural areas, 64% (n=11) identified themselves as Aboriginal or Torres Strait Islanders. Of the overall population, 37% (n=13) were Aboriginal or Torres Strait Islanders, and 63% (n=22), non-Aboriginals (Table 2).
Table 2
| Site | Count of closest surgical margin per site (number) | Surgical margin (mean ± SD), mm |
|---|---|---|
| Anterolateral | 6 | 13.4±5 |
| Lower | 8 | 24.7±8.7 |
| Postcrycoid | 7 | 10.6±4 |
| Posterolateral | 11 | 14.6±4.4 |
| Upper | 3 | 21.8±12 |
| Grand total | 35 | 16.8±2.8 |
SD, standard deviation.
Primary surgery was done in two patients (6%) with T2 disease, three patients (8.5%) with T3 disease, and 19 patients (54%) with T4 disease. Eleven patients (31.5%) were managed with salvage surgery.
Bilateral neck dissection in association with laryngopharyngectomy was done in all our 35 patients. Histopathology report described that more than 18 lymph nodes were resected in all the neck dissections (mean ± SD: 20.7±2.4, 20–21.3 lymph nodes).
In our study, all margins were clear from microscopic disease. Two patients (6%) had close margins between 1 to 2 mm, nine patients (26%) had margins between 10–19 mm and 24 patients (68%) had more than 20 mm. The mean surgical margin was 24.4±16.7 mm (19.3–29.4 mm). The margins were described as anterolateral, posterolateral, postcricoid, upper and lower margin (Table 3). A higher probability of recurrence was identified within patients who had surgical margins <2 mm vs. patients with surgical margins ≥2 mm (81% vs. 19%, P=0.01). The closest margins identified in our study were 1.2 and 1.4 mm into the posterolateral margin. Both patients had stomal recurrence and died from it within 5 years.
Table 3
| Cause of death | Number [%] |
|---|---|
| Disease-specific (n=6) | |
| Stomal site recurrence | 2 [6] |
| Metastatic mediastinal/lung disease | 4 [11] |
| All-cause mortality (n=6) | |
| Malignant bowel obstruction sec to metastatic colorectal cancer | 1 [3] |
| Non-small cell lung cancer | 1 [3] |
| Sepsis other | 4 [11] |
Survival analysis
The overall 5-year mean survival among 35 patients was 59% (5-year mean survival time =4.6 years) (Figure 1).
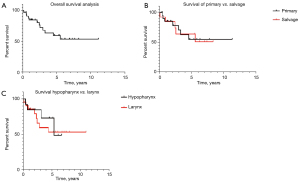
The difference between primary vs. salvage laryngectomy was not significant [5-year mean survival 54% (4.5 years) vs. 63% (5.4 years), P=0.7]; however, patients who had radiotherapy before surgery had more probability of death irrespective of their T status (OR 0.5, 95% CI: 0.13–0.3, P=0.04).
Six patients (17%) died from disease-specific cancers, including two stomal recurrences and four mediastinal/lung metastatic disease (Table 4). Disease-free patients had significantly higher median survival than patients with disease-specific mortality [80.3% (5.2 years) vs. 10% (2.9 years) respectively, P=0.0001].
Table 4
| Characteristics | Overall sample (n=35) | Alive (n=23) | Deceased (n=12) |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis, years | 61.8±3 | 60±3 | 68±4 |
| Female:male | 4%:96% | 1:21 | 1:5 |
| Aboriginal:non-Aboriginal | 1:1.8 | 1:2 | 1:1.4 |
| Cancer site (N=35), n [%] | |||
| Larynx | 24 [68] | 15 [63] | 9 [37] |
| Hypopharynx | 11 [32] | 8 [72] | 3 [28] |
| Primary cancer (N=24), n [%] | |||
| T2 | 2 [5] | 1 [4] | 0 [0] |
| T3 | 3 [16] | 3 [13] | 0 [0] |
| T4 | 19 [79] | 12 [42] | 7 [29] |
| Recurrent cancer (N=11), n [%] | |||
| T2 | 6 [55] | 5 [83] | 1 [17] |
| T3 | 3 [27] | 0 [0] | 3 [100] |
| T4 | 2 [18] | 1 [33] | 2 [67] |
| N stage (N=35), n [%] | |||
| N0 | 25 [71] | 16 [64] | 9 [36] |
| N1 | 1 [3] | 1 [100] | 0 [0] |
| N2 | 7 [20] | 4 [57] | 3 [43] |
| N3 | 2 [6] | 2 [100] | 0 [0] |
| Radiotherapy, n [%] | |||
| Radiation therapy before surgery | 14 [40] | 8 [57.1] | 6 [42.9] |
| NRI malnourishment risk score, n [%] | |||
| Severe risk | 11 [31] | 5 [45] | 6 [55] |
| Moderate risk | 24 [69] | 18 [75] | 6 [25] |
| Indication, n [%] | |||
| Primary laryngectomy | |||
| Hypopharyngeal SCC | 8 [23] | 5 [62] | 3 [38] |
| Laryngeal SCC | 14 [40] | 10 [71] | 4 [29] |
| Salvage laryngectomy | |||
| Laryngeal SCC | 10 [28] | 6 [60] | 4 [40] |
| Stage III and hypopharyngeal SCC | 2 [6] | 1 [50] | 1 [50] |
| Osteoradionecrosis larynx T2 N2a M0 oropharyngeal SCC | 1 [3] | 1 [100] | 0 [0] |
NRI, nutrition risk index; SCC, squamous cell carcinoma.
Severe risk of malnourishment using the NRI score was identified as a contributing factor for complications and higher mortality than those with moderate risk (54% vs. 25%, P=0.01).
Patients with primary laryngeal cancer had a marginally higher probability of death than patients with hypopharyngeal cancer [5-year mean survival 37% (5.4 years) vs. 27% (4.6 years), P=0.05].
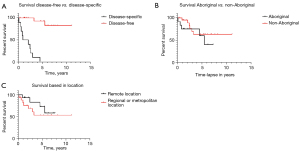
The 5-year disease-specific survival analysis showed similar outcomes for Aboriginal and non-Aboriginal [63% (4.6 years) vs. 63% (5.3 years), P=0.03].
Patients located remotely had a grater survival rate than metropolitan-based [72% (4.7 years) vs. 53% (4.6 years) respectively, P=0.04) (Figure 2).
Complications
Ten patients (28%) developed complications, including 2 (6%) chyle-leaks and 8 (23%) pharyngocutaneous fistulas (PCF). Salvage laryngectomy increased the risk of PCF and chyle leak (OR 0.5, 95% CI: 0.13–0.3, P=0.04).
Hypoalbuminemia (OR 3.2, 95% CI: 1.2–1.6, P=0.01) and severe malnourishment (OR 4.53, 95% CI: 1.234–1.872; P=0.02) were identified as contributing factors of PCF. Preoperative severe malnourishment risk was identified in 28.5% (n=10) of our population (Figure 3).
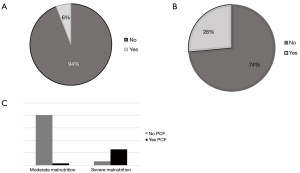
Patients that developed PCF (n=10) were managed with conservative therapy (n=4, 40%), free flap reconstruction (n=2, 20%), and regional flap reconstruction (n=4, 40%).
Discussion
The complex nature of advanced hypopharyngeal and laryngeal malignancies requires a multidisciplinary approach from a head and neck oncology team with relevant expertise. Despite government efforts to close the gap between rural and metropolitan outcomes, most advanced cancers are still managed in subspecialized, large-volume urban centres. The significant barriers for rural patients in accessing these centres potentially delay diagnosis and treatment while adding emotional and financial stressors to the patients (10).
Despite advances in medical and surgical treatment, there has not been a dramatic improvement in patients’ overall survival in advanced stage hypopharyngeal and laryngeal cancer (11). In 2019, Gouzos et al. described an overall survival of 67% over three years in a high-volume centre in Adelaide, Australia (12). Birkeland et al. reported an overall 5-year survival of 49% in a prospective study of 244patients at the University of Michigan Health System (13). Woodard et al. reported an overall survival of 23 months in 143 patients over 5 years at the Loyola University Medical Centre (11). Rodrigues et al. described an overall survival of 25.9% in 5 years in 87 patients at the Hospital da Senhora da Oliveira, Portugal (5). There is no study available at the time of this paper’s publication that compares survival among Aboriginal and non-Aboriginal populations. Our study demonstrated an overall 5-year mean survival of 59.6% (5-year mean survival time of 4.6 years), comparable to national and international survival rates.
Our demographic results show a predominance in male patients living in regional areas in their sixties, consistent with Australia’s reported literature (14). Previous studies had reported a gap between Aboriginal and non-aboriginal stage at presentation and diagnosis; hence, a worse prognosis in survival (15). Ethnicity in our research shows a similar ratio between Aboriginal and non-Aboriginal patients of 1:1.8 on presentation and surgical management. No significant difference was found between Aboriginal [4.6 years (63%)] and non-Aboriginal [5.3 years (63%), P=0.3] groups.
Although international reports describe a substantial survival gap between patients from rural vs. metropolitan areas (10), our results show a grater 5-year mean survival among rurally based patients (72%, 4.7 years) when comparing with metropolitan-based patients (53%, 4.6 years), P=0.01.
Although there is no accepted minimum for LNY in level I–III neck dissection, at least 18 nodes obtained are considered adequate (16). Our study showed that all the neck dissections performed resulted in more than 18 lymph nodes resected (mean ± SD: 20.7±2.4).
Surgical margin clearance has been recognized as an important prognostic factor. Lam et al. described the surgical margins in 70 laryngectomies. Thirteen per cent of their sample had microscopic disease within 1 mm, 6% within 2 mm and 51% within ≥2 mm (17). Our study showed that all margins were clear of microscopic disease. Two patients (6%) had close margins between 1 to 2 mm, nine patients (26%) 10–19 mm, and 24 patients (68%) had more than 20 mm margin.
The presence of surgical complications determines the quality of care and resource utilization. Goepfert et al. describe complications identified in a high-volume institution and their impact on hospital length stay and readmissions within the first 30 days post-surgery. He reported complications occurred in 83 of 245 patients (33.9%), 21.6% wound infection, 13.9% PCF, and 1.2% deaths (18). Our results identified complications in 10 patients (28%), including 2 (6%) chyle-leak and 8 (23%) PCF. No wound infections or deaths were identified in the first 30 days post-surgery. Our data is comparable and slightly lower in the incidence of complications.
PCF after laryngectomy has been recognized as a common and troublesome postoperative complication. Saki et al. reported that the main factors influencing fistula formation are age, gender, smoking, systemic disease, and preoperative radiotherapy (19). Our study has identified two main factors: severe malnourishment identified with an NRI score <83.5 (OR 4.53, 95% CI: 1.234–1.872; P=0.02), and salvage surgery (OR 0.5, 95% CI: 0.13–0.3, P=0.04). Options to prevent these factors include a thorough dietitian assessment and nutrition optimization before surgery and the use of primary preventive flap reconstruction intraoperatively (20).
Conclusions
Small-volume regional centres can make a difference in early detection, treatment compliance, and survival outcomes. This study shows that laryngopharyngectomy surgery in a small-volume Regional Centre can be safe, feasible and effective if the team includes well-trained health professionals who follow national and international standards. Cancer-care in a Regional Centre may avoid financial stress and improve emotional and social well-being. Besides, regional cancer-care offers culturally appropriate care, fundamental to Aboriginal and Torres-strait islander populations.
Our study’s main limitation was its retrospective nature and the limited number of cases available for analysis. The relative scarcity in the literature in high-volume centre studies is consistent, moreover when the present study is based in a low volume centre. A meta-analysis of the results is, hence challenging. A proposal for future research is the generation of a state-wide head and neck cancer database and a prospective study identifying the advantages and challenges of cancer care in a regional centre.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-21-19/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-21-19/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-21-19/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-21-19/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee (TEHREC EC 00153). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Cancer incidence in five continents. Volume IX. IARC Sci Publ 2008;1-837. [PubMed]
- Hall AL, Kromhout H, Schuz J, et al. Laryngeal Cancer Risks in Workers Exposed to Lung Carcinogens: Exposure-Effect Analyses Using a Quantitative Job Exposure Matrix. Epidemiology 2020;31:145-54. [Crossref] [PubMed]
- Groome PA, O’Sullivan B, Mackillop WJ, et al. Laryngeal cancer treatment and survival differences across regional cancer centres in Ontario, Canada. Clin Oncol (R Coll Radiol) 2011;23:19-28. [Crossref] [PubMed]
- Rodrigues J, Breda E, Monteiro E. Surgically-Treated Locoregionally Advanced Hypopharyngeal Cancer: Outcomes. Int Arch Otorhinolaryngol 2018;22:443-8. [Crossref] [PubMed]
- Elliott TE, Elliott BA, Regal RR, et al. Improving rural cancer patients’ outcomes: a group-randomized trial. J Rural Health 2004;20:26-35. [Crossref] [PubMed]
- AIHW. Cancer in Australia 2010: an overview. Canberra: Australian Institute of Health and Welfare, 2010.
- Payne S, Jarrett N, Jeffs D. The impact of travel on cancer patients' experiences of treatment: a literature review. Eur J Cancer Care (Engl) 2000;9:197-203. [Crossref] [PubMed]
- Geographic classifications. 2015. Available online: https://www.ruralhealth.org.au/book/geographic-classifications.
- Smith T. A long way from home: Access to cancer care for rural Australians. Radiography (Lond) 2011;18:38-42. [Crossref]
- Woodard TD, Oplatek A, Petruzzelli GJ. Life after total laryngectomy: a measure of long-term survival, function, and quality of life. Arch Otolaryngol Head Neck Surg 2007;133:526-32. [Crossref] [PubMed]
- Gouzos M, Dale O, Sethi N, et al. Elective neck dissection for the node-negative neck during salvage laryngectomy: an analysis of survival outcomes and complication rates. J Laryngol Otol 2019;133:788-91. [Crossref] [PubMed]
- Birkeland AC, Beesley L, Bellile E, et al. Predictors of survival after total laryngectomy for recurrent/persistent laryngeal squamous cell carcinoma. Head Neck 2017;39:2512-8. [Crossref] [PubMed]
- Head and neck cancers in Australia. In: Reports & data. Australia. 2014. Available online: https://www.aihw.gov.au/reports/cancer/head-neck-cancers-australia/contents/table-of-contents
- Waran E, Zubair MY, O’Connor N. Stage at presentation for head and neck cancer in the Top End of the Northern Territory. Aust J Rural Health 2019;27:177-8. [Crossref] [PubMed]
- Pou JD, Barton BM, Lawlor CM, et al. Minimum lymph node yield in elective level I-III neck dissection. Laryngoscope 2017;127:2070-3. [Crossref] [PubMed]
- Lam KH, Lau WF, Wei WI. Tumor clearance at resection margins in total laryngectomy. Cancer 1988;61:2260-72. [Crossref] [PubMed]
- Goepfert RP, Hutcheson KA, Lewin JS, et al. Complications, hospital length of stay, and readmission after total laryngectomy. Cancer 2017;123:1760-7. [Crossref] [PubMed]
- Saki N, Nikakhlagh S, Kazemi M. Pharyngocutaneous fistula after laryngectomy: incidence, predisposing factors, and outcome. Arch Iran Med 2008;11:314-7. [PubMed]
- Chee N, Siow JK. Pharyngocutaneous fistula after laryngectomy--incidence, predisposing factors and outcome. Singapore Med J 1999;40:130-2. [PubMed]
Cite this article as: Reyes-Chicuellar N, Loh TL, Patel H. Laryngopharyngectomy surgery in a small volume regional centre—is it safe, feasible, and effective? Aust J Otolaryngol 2022;5:16.


