
Elective neck dissection improves regional control in early oral squamous cell carcinoma
Introduction
In 2020 there were approximately 930,000 new cases of head and neck cancer (HNC) globally (1). Of these approximately 380,000 cases were from cancers of the oral cavity, including the lip, making it the 18th most common cancer worldwide (1). Australian data from 2017 reported an approximate incidence 3,877 HNC, of which 1,649 were oral cancer (excluding lip) including 1,005 tongue cancers (2). Oral squamous cell carcinoma (OSCC) accounts for 90% of cancers of the oral cavity (3).
Oral cavity subsites include the oral tongue, floor of mouth (FOM), labial and buccal mucosa, maxillary and mandibular gingiva, retromolar trigone and hard palate (4). The outcomes vary depending on disease stage, subsite location, patient age and other patient factors (5). The 3- and 5-year survival rates with early stage OSCC (T1/T2 N0) are 92.2% and 75–89% respectively (6-9). Oral tongue is the most common subsite globally and in Australia and is associated with poorest prognosis and highest rates of recurrence (10-12).
Surgical resection remains the primary treatment modality for early stage OSCC with adjuvant therapy dependent on pathological factors (4,5). The role of elective neck dissection (END) in patients with early OSCC (T1/T2) and clinically negative neck (clinical N0) is controversial (13-18). Three different strategies for the management of a clinically negative neck have been described; observation (Obs) (clinically and/or ultrasonographically), END and sentinel node biopsy (18).
Some have argued that END in this cohort does not provide a survival or recurrence benefit when compared with Obs (with salvage therapy if required) (14,19,20). More recent randomized prospective studies have demonstrated significant survival and recurrence benefits with END (16,17,21). END in clinically negative necks have shown rates of occult metastases of approximately 30% (22-26). Increased depth of invasion (DOI) has been demonstrated to increase the risk of occult metastasis and there is also significant subsite variability, with FOM cancers being higher risk than oral tongue at a comparable DOI for example (22,26,27).
This paper aims to describe the treatment, outcomes, and their changes over time of a cohort of early OSCC patients treated in an Australian tertiary HNC referral centre. We present this article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-22-20/rc).
Methods
Cohort selection
The Sydney Head and Neck Cancer Institute (SHNCI) has maintained a prospective database of HNC patients since 1987. Patients with T1N0 and T2N0 OSCC from the 1st January 1987 to 31st March 2019, reviewed and restaged to American Joint Committee on Cancer (AJCC) 8th edition staging, were identified retrospectively for inclusion. N0 was defined as clinically N0 for patients who did not undergo END or pathologically N0 for those who underwent END. Lip as a subsite was not included. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Sydney Local Health District Ethics and Review Committee (protocol X16-0367). Individual consent for this retrospective analysis was waived.
Data collection and review
Patient demographic, histopathological reports, treatment and follow-up data were extracted from the database on the 10th April 2019. Missing datapoints were filled following a review of histopathology and patient records.
Statistical analysis
The cohort was divided into three time-brackets: 1987–1999, 2000–2010 and 2011–2019, for analysis of trends over time. Treatment factors of interest were END and radiotherapy. The endpoints evaluated were regional control, disease-specific survival (DSS) and overall survival (OS). Recurrence status was determined by a combination of clinical examination and imaging studies. Local recurrence was defined as recurrent disease at same oral subsite as the primary OSCC. Regional recurrence was defined as recurrent disease in the neck. Distant recurrence was defined as recurrent disease at a site other than the head and neck. Regional control was determined from date of initial surgery to date of recurrence, or to the date of last follow-up or death. DSS was determined from date of initial surgery to date of last follow-up or date of death from disease. OS was determined from date of initial surgery to date of last follow-up or death. Statistical analysis was performed with IBM SPSS Statistics 20. Univariate and multivariate analysis was carried out using Cox proportional hazards regression. Kaplan-Meier (KM) survival curves were produced where applicable.
Results
Cohort details
The cohort of early OSCC identified from the database consisted of 391 patients, which was reduced to 377 after review of patient records and histopathology resulted in exclusion of 14 patients as depicted in Figure 1. The mean age of the cohort was 62.0±12.9 years. There were 212 (56.2%) male patients and 165 (43.8%) female patients. At the time of review 160 of the cohort were deceased, with 32 (8.5%) of them confirmed deceased from OSCC. Median follow-up period was 3.8 years (1 day–25.1 years). Of the 377 patients in the cohort 230 (61.0%) were T1 and 147 (39.0%) were T2. Oral tongue was the most common subsite with 223 cases, accounting for 59.2% of the entire cohort. Further cohort summary details are outlined in Table 1.
Table 1
| Variable | No. of patients (%) |
|---|---|
| Sex | |
| Male | 212 (56.2) |
| Female | 165 (43.8) |
| Pathological variables | |
| T stage | |
| T1 | 230 (61.0) |
| T2 | 147 (39.0) |
| Differentiation | |
| Well | 80 (21.2) |
| Moderate | 187 (49.6) |
| Poor | 33 (8.8) |
| Unknown | 77 (20.4) |
| Margin | |
| Involved | 22 (5.8) |
| Close | 239 (63.4) |
| Clear | 108 (28.6) |
| Unknown | 8 (2.1) |
| Perineural invasion | 42 (11.1) |
| T1 | 12 (3.1) |
| T2 | 30 (8.0) |
| Multifocal | 9 (8=T2, 1=T1) (2.4) |
| Lymphovascular invasion | 30 (8.0) |
| T1 | 9 (2.4) |
| T2 | 21 (5.6) |
| Depth of invasion | |
| 0–5 mm | 210 (55.7) |
| >5 mm | 113 (30.0) |
| Unknown | 54 (14.3) |
| Treatment | |
| Neck dissection | 183 (48.5) |
| T1 neck dissection | 73 (19.4) |
| T2 neck dissection | 110 (29.2) |
| Radiotherapy | 41 (10.9) |
| Outcomes | |
| All deaths | 160 (42.4) |
| Death from OSCC | 32 (8.5) |
| Recurrence | 95 (25.2) |
| Local | 54 (14.3) |
| Regional | 35 (9.3) |
| Distant | 6 (1.6) |
| Salvage of regional recurrence | Total 35* |
| Surgery | 28 (80.0*) |
| Radiotherapy | 24 (68.6*) |
| Surgery + radiotherapy | 20 (57.1*) |
*, percentage of salvage subtotal. OSCC, oral squamous cell carcinoma.
Treatment trends
All 377 patients received surgical resection of the primary tumour. END was performed on 183 (48.5%) of the patients. Of these OSCC cases, 73 were T1 and 110 were T2. An increased rate of END over time was apparent with 35.6% in 1987–1999 group, 49.2% in 2000–2010 group and 57.4% in 2011–2019 group (P=0.003) and is summarized in Table 2. Radiotherapy was administered as an adjuvant therapy in 41 patients, accounting for 10.9% of the cohort. The rationale for adjuvant radiotherapy was determined by identification of high-risk histopathological features and discussed in a multi-disciplinary team setting. The specific high-risk features have evolved with the evolution of the evidence base but include features such as margin status, DOI, perineural and lymphovascular invasion. Of those receiving radiotherapy, 6 were T1 and 35 were T2 cases. There was no statistically significant change in radiotherapy administration over time (P=0.42).
Table 2
| Period | No. of neck dissection (% of period) | Total |
|---|---|---|
| Total | 183 | 377 |
| 1987–1999 | 37 (35.58) | 104 |
| T1 | 16 (15.39) | |
| T2 | 21 (20.19) | |
| 2000–2010 | 65 (49.24) | 132 |
| T1 | 23 (17.42) | |
| T2 | 42 (31.82) | |
| 2011–2019 | 81 (57.45) | 141 |
| T1 | 34 (24.12) | |
| T2 | 47 (33.33) |
Recurrence rates and sites
A total of 95 instances of recurrence were identified, accounting for 25.2% of the cohort. Local recurrence occurred in 54 cases, regional recurrence in 35 and distant recurrence in 6 cases. These recurrence sites were not mutually exclusive.
Treatment factors and prognosis
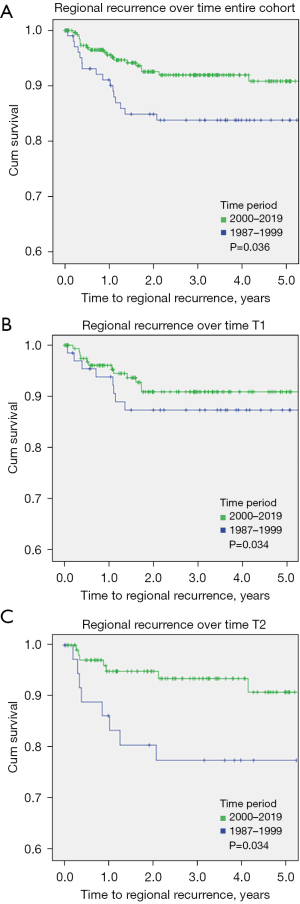
Rates of regional recurrence was worse in patients in the 1987–1999 group when compared with those treated 2,000 onwards (P=0.036) as shown in Figure 2A. There was no difference in regional recurrence demonstrated when comparing T1 OSCC only as shown in Figure 2B. This difference in regional recurrence was more profound when comparing the T2 subgroups only (P=0.034) and is shown in Figure 2C. There was no significant trend in DSS (P=0.83) or OS (P=0.19) over time demonstrated.
Of the 183 patients with END, 27 (14.8%) required salvage surgery and 8 (4.4%) required both salvage surgery and radiotherapy. Of the patients managed with Obs, 49 (25.3%) required salvage surgery and 23 (11.9%) required both salvage surgery and radiotherapy.
Of the 35 patients who had a regional recurrence, 28 (80.0%) underwent salvage surgery and 20 (57.1%) received both salvage surgery and radiotherapy. Importantly, for patients who received salvage therapy (surgery and/or radiotherapy), there was no difference in DSS (P=0.79) or OS (P=0.98) between END or Obs groups.
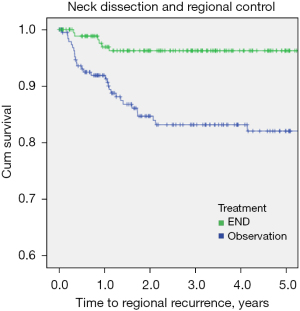
For this cohort, patients with END demonstrated a significant reduction in regional recurrence (HR 0.21, P<0.001) as shown in Table 3 and Figure 3. On multivariable Cox analysis, the decision for END was an independent predictor for regional control (P=0.001), after adjusting for age, DOI and PNI (Table 3). Whilst, END resulted in better regional control, it did not translate into improved DSS nor OS, with an estimated 5-year OS of 71% vs. 71% (P=0.86) for those with and without END respectively and an estimated 5-year DSS of 87% vs. 94% for those with and without END respectively (P=0.11).
Table 3
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Age | 1.03 | 1.01–1.06 | 0.019 | 1.03 | 1.01–1.06 | 0.022 | |
| PNI | 1.93 | 0.80–4.64 | 0.145 | ||||
| DOI >5 mm | 1.89 | 0.98–3.69 | 0.059 | ||||
| Neck dissection | 0.21 | 0.09–0.50 | <0.001 | 0.23 | 0.10–0.56 | 0.001 | |
| RT | 1.16 | 0.64–2.10 | 0.623 | ||||
| Time period | 0.50 | 0.26–0.97 | 0.040 | 0.58 | 0.29 | 0.112 | |
CI, confidence interval; PNI, perineural invasion; DOI, depth of invasion; RT, radiotherapy.
Discussion
This study describes a cohort of early OSCC (T1/T2N0) patients and the treatment variables that impact recurrence and survival over a 32-year period. With a cohort of 377 this is the largest study of this type to have been conducted in Australia to our knowledge.
As stated previously, the role of END in early OSCC with a clinically negative neck has been a controversial topic, with diversity of opinion and evidence across the world (15,18,28,29). Historically, much of the literature exploring the role of END has involved retrospective analysis of early OSCC, and has often failed to reach consensus on the matter (20,29-36). A limiting factor in the broad utility of these studies has been the selection bias in which patients have been managed with END. This is largely due to the appropriate advances in risk stratification with the inclusion of histopathological features such as DOI and perineural invasion, resulting in patients with high-risk tumours receiving END. More recently, randomized, prospective, controlled trials by D’Cruz et al. and Hutchison et al., have demonstrated clear survival and recurrence benefits for END when compared to Obs (14,16).
In this study cohort, a clear increasing trend of END in the management of early OSCC was demonstrated. The likelihood of undergoing a neck dissection between 2000–2009 was 1.5 times higher than the preceding decade, with the trend continuing to 2.4 times higher likelihood between 2010–2019. In 2003, O’Brien et al. established the 4 mm thickness cutoff as a powerful discriminator in early OSCC for determining risk of local control, nodal disease and survival rates (37). The inclusion of this parameter in the clinical decision-making process is likely responsible for the increasing rate of neck dissections from the early 2000s onwards at this centre. It is important to note that there was no change in the DOI observed over these time periods (P=0.253).
A significant reduction in the rate of regional recurrences was also demonstrated over this time in those who received an END (P≤0.001). This supports the growing consensus in the literature of the regional control benefits of neck dissection for early OSCC (16,17,22,32). In an earlier, comparable Australian cohort, Lin et al. demonstrated a similar regional control benefit when performing END in T1/T2N0 oral tongue SCC (32).
Interestingly, neck dissection in this cohort did not demonstrate statistically significant benefit for DSS or OS, which has been demonstrated elsewhere. On further assessment of the cohort, we believe this is explained by the high rates of salvage surgery (80%) and surgery and radiotherapy (68.6%) in correcting the survival outcomes for patients with neck recurrence and is supported by the literature (19,38). For salvage therapy to be provided, timely detection of recurrent disease is necessary which introduces another integral and topical component of OSCC management: surveillance interval and method.
Whilst not specifically recorded for this cohort, this unit’s standard practice to provide follow-up every 2–3 months post-surgery initially, with decreasing frequency of visits for a surveillance period of up to 5 years where possible. The benefit of ultrasound imaging in early OSCC surveillance has been demonstrated in the literature (19,39,40). Salvage rates of up 80–100% have been reported by several institutions, by employing frequent clinical examination and ultrasound surveillance in the follow-up period (19,40). We believe similarly intensive surveillance on this study cohort may explain both the high salvage rates and comparable survival outcomes between the END and Obs groups. It must also be recognised that the application of similar surveillance programs in other clinical settings may not be viable from a clinician and cost perspective.
The disease specific mortality from OSCC in this cohort was 8.5%, which is substantially lower than that presented by Hutchison et al. (19.6%). D’Cruz et al. did not outline disease specific mortality but did report worse OS when compared to Hutchison et al. (16,21). This discrepancy does raise the potential issue of the generalizability of these RCT findings to the Australian context, where different patient demographics, disease patterns and health-care systems may come into play.
Salvage surgery in HNC has been demonstrated to improve locoregional control and survival (16,38). It has however also been recognized as requiring more extensive surgery and adjuvant therapy when compared with END and primary lesion resection and consequently may be associated with greater physical and psychological morbidity (16,38,41-44). The morbidity associated with END is predominantly related to neck motor and sensory nerve impairment and swallowing difficulties (16). While these are often considered low-grade, they still impact quality of life. This must be weighed with the comparative morbidity associated with salvage surgery or other modalities, which can be arguably worse than that of an END (16,42). Post-operative morbidity data was unfortunately not recorded in this dataset and further commentary regarding this with respect to this study’s cohort is challenging.
This cohort demonstrated a local recurrence rate of 13.0% and a regional recurrence rate of 9.0%. This compares favourably to similar cohorts in the literature, with rates of 11–34% local recurrence and 18–31% regional recurrence reported (6,32,45,46). This finding along with 8.5% of the cohort dying from OSCC reinforces the importance of intensive surveillance and early intervention, even in this early disease group.
Strengths of this study include the large number of patients included in the cohort and the management of the cohort at a tertiary referral unit with significant multidisciplinary input. This may also be considered a potential limitation in the generalizability of the reported findings to other clinical settings. Weaknesses of the study include its retrospective nature and the associated selection bias likely introduced in patients who have adverse pathological features being more likely to undergo END. Another limitation was missing data points for historical histopathological assessments, although the impacts of this on conclusions drawn from this study are minimal.
Conclusions
This study describes a large cohort of early OSCC managed at a tertiary cancer referral centre in Sydney, Australia. We observed a trend of increasing use of END in the management of early OSCC over the last 3 decades, which is in keeping with global trends and influenced by improved risk stratification. This increasing use of END is associated with improved regional control. We are confident this study supports the growing consensus of the role of END in the management of early OSCC. Reassuringly, we have also demonstrated that in the event of regional recurrence in patients who are initially managed with Obs, salvage therapies can maintain comparable survival outcomes to patients who undergo END.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-22-20/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-22-20/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-22-20/prf
Conflicts of Interest: All authors have completed the ICMJE’s unified disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-22-20/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Sydney Local Health District Ethics and Review Committee (protocol X16-0367). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Australian Institute of Health Welfare. Cancer data in Australia. Canberra: AIHW; 2021.
- El-Naggar AK, Chan JKC, Rubin Grandis J, et al. WHO classification of head and neck tumours. 4th edition. ed. Lyon: International Agency for Research on Cancer; 2017.
- Almangush A, Mäkitie AA, Triantafyllou A, et al. Staging and grading of oral squamous cell carcinoma: An update. Oral Oncol 2020;107:104799. [Crossref] [PubMed]
- Chinn SB, Myers JN. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J Clin Oncol 2015;33:3269-76. [Crossref] [PubMed]
- Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue--clinicopathologic features affecting outcome. Cancer 2012;118:101-11. [Crossref] [PubMed]
- Cheraghlou S, Schettino A, Zogg CK, et al. Changing prognosis of oral cancer: An analysis of survival and treatment between 1973 and 2014. Laryngoscope 2018;128:2762-9. [Crossref] [PubMed]
- Amit M, Yen TC, Liao CT, et al. Improvement in survival of patients with oral cavity squamous cell carcinoma: An international collaborative study. Cancer 2013;119:4242-8. [Crossref] [PubMed]
- Liu TPJ, Fisher BM, Chua B, et al. Survival outcomes following modern multidisciplinary management of oral squamous cell carcinoma in Australia. Oral Surg Oral Med Oral Pathol Oral Radiol 2021;131:92-8. [Crossref] [PubMed]
- Miranda-Filho A, Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol 2020;102:104551. [Crossref] [PubMed]
- Ng JH, Iyer NG, Tan MH, et al. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck 2017;39:297-304. [Crossref] [PubMed]
- Almangush A, Bello IO, Coletta RD, et al. For early-stage oral tongue cancer, depth of invasion and worst pattern of invasion are the strongest pathological predictors for locoregional recurrence and mortality. Virchows Arch 2015;467:39-46. [Crossref] [PubMed]
- Rahman N, Conn B. Evaluation of Histopathological Risk Model in a Cohort of Oral Squamous Cell Carcinoma Patients Treated with Accompanying Neck Dissection. Head Neck Pathol 2021;15:1156-61. [Crossref] [PubMed]
- D'Cruz AK, Siddachari RC, Walvekar RR, et al. Elective neck dissection for the management of the N0 neck in early cancer of the oral tongue: need for a randomized controlled trial. Head Neck 2009;31:618-24. [Crossref] [PubMed]
- Govers TM, de Kort TB, Merkx MA, et al. An international comparison of the management of the neck in early oral squamous cell carcinoma in the Netherlands, UK, and USA. J Craniomaxillofac Surg 2016;44:62-9. [Crossref] [PubMed]
- Hutchison IL, Ridout F, Cheung SMY, et al. Nationwide randomised trial evaluating elective neck dissection for early stage oral cancer (SEND study) with meta-analysis and concurrent real-world cohort. Br J Cancer 2019;121:827-36. [Crossref] [PubMed]
- Ren ZH, Xu JL, Li B, et al. Elective versus therapeutic neck dissection in node-negative oral cancer: Evidence from five randomized controlled trials. Oral Oncol 2015;51:976-81. [Crossref] [PubMed]
- Vassiliou LV, Acero J, Gulati A, et al. Management of the clinically N(0) neck in early-stage oral squamous cell carcinoma (OSCC). An EACMFS position paper. J Craniomaxillofac Surg 2020;48:711-8. [Crossref] [PubMed]
- Yuen AP, Ho CM, Chow TL, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck 2009;31:765-72. [Crossref] [PubMed]
- Liu TR, Chen FJ, Yang AK, et al. Elective neck dissection in clinical stage I squamous cell carcinoma of the tongue: Does it improve regional control or survival time? Oral Oncol 2011;47:136-41. [Crossref] [PubMed]
- D'Cruz AK, Vaish R, Kapre N, et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N Engl J Med 2015;373:521-9. [Crossref] [PubMed]
- Ding Z, Xiao T, Huang J, et al. Elective Neck Dissection Versus Observation in Squamous Cell Carcinoma of Oral Cavity With Clinically N0 Neck: A Systematic Review and Meta-Analysis of Prospective Studies. J Oral Maxillofac Surg 2019;77:184-94. [Crossref] [PubMed]
- Anand A, Vidhyadharan S, Subramaniam N, et al. Selective Neck Dissection in Oral Cavity Cancer Is Not Without Morbidity. Indian J Surg Oncol 2021;12:5-11. [Crossref] [PubMed]
- Fasunla AJ, Greene BH, Timmesfeld N, et al. A meta-analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node-negative neck. Oral Oncol 2011;47:320-4. [Crossref] [PubMed]
- Huang SF, Kang CJ, Lin CY, et al. Neck treatment of patients with early stage oral tongue cancer: comparison between observation, supraomohyoid dissection, and extended dissection. Cancer 2008;112:1066-75. [Crossref] [PubMed]
- Keski-Säntti H, Atula T, Törnwall J, et al. Elective neck treatment versus observation in patients with T1/T2 N0 squamous cell carcinoma of oral tongue. Oral Oncol 2006;42:96-101. [Crossref] [PubMed]
- Balasubramanian D, Ebrahimi A, Gupta R, et al. Tumour thickness as a predictor of nodal metastases in oral cancer: comparison between tongue and floor of mouth subsites. Oral Oncol 2014;50:1165-8. [Crossref] [PubMed]
- van Lanschot CGF, Klazen YP, de Ridder MAJ, et al. Depth of invasion in early stage oral cavity squamous cell carcinoma: The optimal cut-off value for elective neck dissection. Oral Oncol 2020;111:104940. [Crossref] [PubMed]
- Kelner N, Vartanian JG, Pinto CA, et al. Does elective neck dissection in T1/T2 carcinoma of the oral tongue and floor of the mouth influence recurrence and survival rates? Br J Oral Maxillofac Surg 2014;52:590-7. [Crossref] [PubMed]
- Nguyen E, McKenzie J, Clarke R, et al. The Indications for Elective Neck Dissection in T1N0M0 Oral Cavity Squamous Cell Carcinoma. J Oral Maxillofac Surg 2021;79:1779-93. [Crossref] [PubMed]
- Wushou A, Wang M, Yibulayin F, et al. Patients With cT1N0M0 Oral Squamous Cell Carcinoma Benefit From Elective Neck Dissection: A SEER-Based Study. J Natl Compr Canc Netw 2021;19:385-92. [Crossref] [PubMed]
- Lin MJ, Guiney A, Iseli CE, et al. Prophylactic neck dissection in early oral tongue squamous cell carcinoma 2.1 to 4.0 mm depth. Otolaryngol Head Neck Surg 2011;144:542-8. [Crossref] [PubMed]
- Pugazhendi SK, Thambiah L, Venkatasetty A, et al. Elective neck dissection versus "wait and watch" policy in tongue carcinoma. J Pharm Bioallied Sci 2012;4:S226-9. [Crossref] [PubMed]
- Tai SK, Li WY, Chu PY, et al. Risks and clinical implications of perineural invasion in T1-2 oral tongue squamous cell carcinoma. Head Neck 2012;34:994-1001. [Crossref] [PubMed]
- Feng Z, Cheng A, Alzahrani S, et al. Elective Neck Dissection in T1N0M0 Oral Squamous Cell Carcinoma: When Is It Necessary? J Oral Maxillofac Surg 2020;78:2306-15. [Crossref] [PubMed]
- Peng KA, Chu AC, Lai C, et al. Is there a role for neck dissection in T1 oral tongue squamous cell carcinoma? The UCLA experience. Am J Otolaryngol 2014;35:741-6. [Crossref] [PubMed]
- O'Brien CJ, Lauer CS, Fredricks S, et al. Tumor thickness influences prognosis of T1 and T2 oral cavity cancer--but what thickness?. Head Neck 2003;25:937-45. [Crossref] [PubMed]
- van den Bovenkamp K, Noordhuis MG, Oosting SF, et al. Clinical outcome of salvage neck dissections in head and neck cancer in relation to initial treatment, extent of surgery and patient factors. Clin Otolaryngol 2017;42:693-700. [Crossref] [PubMed]
- Dunsky KA, Wehrmann DJ, Osman MM, et al. PET-CT and the detection of the asymptomatic recurrence or second primary lesions in the treated head and neck cancer patient. Laryngoscope 2013;123:2161-4. [Crossref] [PubMed]
- Flach GB, Tenhagen M, de Bree R, et al. Outcome of patients with early stage oral cancer managed by an observation strategy towards the N0 neck using ultrasound guided fine needle aspiration cytology: No survival difference as compared to elective neck dissection. Oral Oncol 2013;49:157-64. [Crossref] [PubMed]
- Malone J, Robbins KT. Neck dissection after chemoradiation for carcinoma of the upper aerodigestive tract: indications and complications. Curr Opin Otolaryngol Head Neck Surg 2010;18:89-94. [Crossref] [PubMed]
- Ho AS, Kraus DH, Ganly I, et al. Decision making in the management of recurrent head and neck cancer. Head Neck 2014;36:144-51. [Crossref] [PubMed]
- Hermann RM, Christiansen H, Rödel RM. Lymph node positive head and neck carcinoma after curative radiochemotherapy: a long lasting debate on elective post-therapeutic neck dissections comes to a conclusion. Cancer Radiother 2013;17:323-31. [Crossref] [PubMed]
- Haddadin KJ, Soutar DS, Oliver RJ, et al. Improved survival for patients with clinically T1/T2, N0 tongue tumors undergoing a prophylactic neck dissection. Head Neck 1999;21:517-25. [Crossref] [PubMed]
- Hicks WL Jr, North JH Jr, Loree TR, et al. Surgery as a single modality therapy for squamous cell carcinoma of the oral tongue. Am J Otolaryngol 1998;19:24-8. [Crossref] [PubMed]
- Sessions DG, Spector GJ, Lenox J, et al. Analysis of treatment results for oral tongue cancer. Laryngoscope 2002;112:616-25. [Crossref] [PubMed]
Cite this article as: Beddow T, Low TH, Gao K, Wykes J, Gupta R, Clark J, Elliott M. Elective neck dissection improves regional control in early oral squamous cell carcinoma. Aust J Otolaryngol 2023;6:16.


