The impact of anticoagulation on management of anterior epistaxis: retrospective case series
Introduction
With the emergence of coronavirus disease 2019 (COVID-19) pandemic and rise in presentations to the emergency department (ED), an unprecedented demand on healthcare resources challenged the management of all illnesses.
Epistaxis is common with 60% of adults experiencing it over their lifetime (1), but only 10% require medical treatment (2). It accounts for 0.5% of all presentations to ED (3-5), who stay in hospital for 2.9 days on average (6).
Multiple therapeutic algorithms are proposed with good data on efficacy of each, but outcome comparison is lacking because of the heterogeneity of patient population and variability of treatment choices (4).
The incidence of epistaxis is higher with anticoagulation therapy (ACT). Their use is a significant risk factor for both recurrent and intractable disease (7,8). It increases the need for nasal packing or surgical intervention and increases length of stay in hospital (LOS) (9). But not all types of ACT worsen the outcome (10).
ACT and antiplatelet therapy (APT) are the standard of care for prevention and treatment of many cardiovascular disease (CVD). With an increasing aging population, their use is on the rise (11-15). The new direct oral anticoagulants (DOACs) work directly on the coagulation cascade and have become the gold standard of treatment due to superiority to Warfarin (16-20). However, DOACs are not risk free. They have been associated with an increased risk of extracranial bleed (12). Prior to the availability of the reversal agent, García Callejo (in 2014) reported 1,000 deaths on dabigatran, of which 14.8% were secondary to epistaxis (21). Despite the availability of reversal agents, the risk remains considerable, as access to these are limited (22).
This study aims to analyse the impact of ACT on outcomes of epistaxis in patients presented to Australia’s largest multicentre tertiary care otolaryngology service. COVID pandemic impacts the data as it emerged, and its impact was reviewed. Furthermore, a comprehensive review of literature was performed to compare the findings. We present this article in accordance with the STROBE reporting checklist (available at: https://www.theajo.com/article/view/10.21037/ajo-23-18/rc).
Methods
Study design
The study is a retrospective case series of patients presented with epistaxis to the three ED under Monash Health umbrella, which received patients from large number of regional, rural, or remote service providers.
The data was captured from the computerised medical record program (Cerner) from 1st of August 2019 to 1st of August 2020 (1 year). Missing data was left blank.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Monash Health research office, the local human research ethics committee (QA/69710/MonH-2021-263665). Because of the nature of this audit, the requirement for informed consent was waived.
Study eligibility
Epistaxis was defined as any amount of bleeding from nasal/sinonasal space that required hospital attendance.
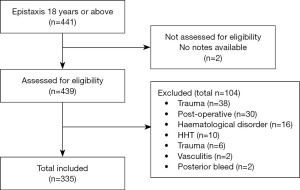
Any patient who was above 18 years old, with no history of trauma or sinonasal surgery within 6 weeks prior to presenting was included. Anyone with sinonasal tumour, bleeding disorders, vasculitis, and hereditary haemorrhagic telangiectasia. Posterior epistaxis was considered any bleed identified posterior to pyriform aperture or documented as “posterior bleed” by the clinician in the medical record. All posterior epistaxis were excluded (Figure 1).
Data extraction
The consecutive data was collected on demographics of patient (including age and sex), vitals on presentation [including record of blood pressure (BP) and heart rate (HR)], status of ACT/APT; comorbidities [including hypertension (HT), diabetes mellitus (DM), CVD, renal or liver disease, chronic obstructive pulmonary disease, asthma, obstructive sleep apnea (OSA), and recurrent epistaxis]; as well as treatment administered. For those who presented after the first confirmed case of COVID in Melbourne, Australia (19th of January 2020), COVID data including testing on admission and COVID status throughout admission, were also recorded.
The advanced trauma life support classification of shock was used to stratify the severity of bleeding. When the blood loss volume was not available surrogate indicator such as Hb with haemoglobin mass loss formula was used (23). The exact quantity of blood loss volume was limited by the missing data, so the results broadly reported as major and minor. Class III and IV were defined as a major bleed (24).
HT was defined as anyone with a formal diagnosis of HT or documented systolic BP (SBP) or diastolic greater than 135 or 80 respectively, on more than one occasion. Tachycardia was defined as HR greater than 100 bpm.
Treatment types included: topical ointment - vaseline, paraffin and kenacomb; saline sprays; vasoconstrictors; antifibrinolytic agents (TXA), cautery (chemical/electrocautery), packing [dissolvable packing (DP)/non-dissolvable packing (NDP)], cessation of ACT/APT, interventions (embolization or surgery), and patient education.
Treatment outcome data included: duration of each treatment, LOS, success, recurrence, morbidity, and mortality.
Study endpoints
Outcomes data were classified to clinical (treated, recurred, sustained complications) and episodes (LOS, recurrent admission). Success of treatment was defined as no recurrent epistaxis or adverse events. Recurrent admission was defined as any admission within 30 days of treatment. Recurrent epistaxis was divided into short term (<6 weeks) and long-term (>6 weeks but no more than 12 months). The primary outcome was divided into short-term and long-term success. The secondary outcome was the LOS.
Statistical analysis
Microsoft Excel (Washington, USA) was used to compile data and analysed with SPSS version 28.0.1 (IBM, NY, USA) and STAT (NC, USA) (17). P<0.05 is considered significant. Univariant and multivariant analysis confirmed the potential risk factors for failure. Logistic regression applied to assess the treatment outcome in short-term and long-term.
Results
Literature search
A total of 220 literatures met the inclusion criteria, including 8 guidelines. 29 articles focused on the effect of ACT with or without APT, 26 on topical treatments, 29 on effectiveness of packing, 24 on cautery, 17 on relationship with HT, and 13 on recurrent epistaxis.
Data analysis
Total of 257 patients met the inclusion criteria; 47 patients presented twice and 11 more than twice. Therefore, overall database included 335 episodes of admission. Nasal packing was inserted more than once in some admissions, therefore total 342 nasal packing.
Comorbidities
A total of 117 patients were older than 69 years old. This age limit was identified as the significant age with a significant increased risk for incidence and recurrence of epistaxis in the literature.
A total of 163 patients had HT. Fourteen presented with HT crisis (SBP >200 mmHg). An additional 121 BP were high (SBP >135 or diastolic blood pressure >85 mmHg) on arrival to ED but did not have diagnosis of HT. The high BP recordings were not followed up to establish what percentage of 121 patients may have undiagnosed HT. HT was more common in those with recurrent epistaxis (49 out of 73).
Regarding other co-morbidities, 84 (32.7%) of patients had CVD including, 69 (26.8%) had AF, 50 (19.5%) had DM and 14 (5.4%) had renal disease.
Anticoagulation/antiplatelet
In regard to ACT and APT use, 59 (23.0%) patients were on ACT, of which 47 were on DOAC, 12 on warfarin, whilst 72 (28.0%) patients were on APT, of which 3 were on clopidogrel only, 14 on aspirin only and 4 on dual APT. Only 11 patients were on combination of ACT and APT.
In those who presented with recurrent epistaxis, 44 (61.0%) were on either ACT or APT. These included 6 on aspirin, 1 on clopidogrel, 1 on dual therapy, 3 on ACT (all on DOAC) and only 1 on combination of APT and ACT.
Eleven out of 19 patients on Warfarin had recorded international normalized ratio (INR). INR was above the therapeutic range (4.0–5.1) in 5 episodes (out of 11). INR and Coag results were not reported on patients on DOAC or not on any anticoagulation.
Severity of bleeding
Severe bleeding was identified in 3 of 335 episodes (all Class III) (24).
Treatment type and effect
The commonest treatment type was cautery with silver nitrate (n=113, 44.0%). Where patients were packed, equal number (n=28, 10.9%) received NDP or DP. Twelve patients (4.7%) received combination of the two (NDP followed by removal and insertion of DP). Ointment was used in 87 (33.9%), and education completed for only 81 (31.5%).
Thirty-six treatment combinations were identified. Since there were many treatment combinations, the number within some groups were too small to find a meaningful comparison. Therefore, logistic regression was applied to analyse the treatment effect.
Univariant and multivariant analysis followed by logistic regression identified age, HR and HT as the factors with significant impact on the outcome (recurrence/increase LOS).
Sixty-nine patients represented within 30 days of treatment, of these 47 patients represented twice and 11 more than twice. The LOS ranged from 1 to 12.5 days, but majority (91.9%) admitted for 1 day.
Patients who were not on ACT or APT had 80% success in stopping epistaxis. However, patients on ACT or APT had 62.1% and 66.2% success in controlling epistaxis. In those on both APT and ACT, the success was reduced to 45.0%.
A greater percentage of patients who received non-absorbable nasal packing had a successful outcome (75.17%) compared to those who received absorbable nasal packing (45.83%) (Table 1). If the patient was on ACT or APT or both, absorbable nasal packing was significantly associated with a greater frequency of unsuccessful outcome (60.61%), compared to those who were receive NDP (32.37%) (Table 2). The sample size in both chi-square tests were small, and although the result in Table 2, are significant, the identified association was further analysed with logistic regression to control for the variables to further confirm the significance of this association. The analysis was checked for bias. No bias was found.
Table 1
| Packing type | No success, n (%) | Successful, n (%) | Total, n (%) |
|---|---|---|---|
| NDP | 73 (24.83) | 221 (75.17) | 294 (100.00) |
| DP (Nasopore) | 26 (54.17) | 22 (45.83) | 48 (100.00) |
| Total | 99 (28.95) | 243 (71.05) | 342 (100.00) |
Pearson chi2 =17.2662, P=0.000. NDP, non-dissolvable packing; DP, dissolvable packing.
Table 2
| Packing type | No success, n (%) | Successful, n (%) | Total, n (%) |
|---|---|---|---|
| NDP | 45 (32.37) | 94 (67.63) | 139 (100.00) |
| DP (Nasopore) | 20 (60.61) | 13 (39.39) | 33 (100.00) |
| Total | 65 (37.79) | 107 (62.21) | 172 (100.00) |
Pearson chi2 =9.0415, P=0.003. NDP, non-dissolvable packing; DP, dissolvable packing.
Logistic regression controlled for comorbidities with significant impact on the outcome (age, HR and HT). It analysed both short-term and long-term outcome.
In the short-term (<6 weeks), the ACT/APT group overall had lower odds of success. Cautery was associated with lowering the odds of success in all groups. This association was significant for those not on ACT/APT but only trending for those on ACT/APT.
Those who did not receive packing had significantly better odds of success than those received DP (Nasopore). However, majority of patients had cautery prior packing; and sample size was small. A well-designed controlled study can establish the cost-effectiveness of DP (Table 3).
Table 3
| Treatment combination | Odd ratio | Std. Err | z | P>z | 95% CI |
|---|---|---|---|---|---|
| Reference: no cautery, no ACT/APT | 1.00 | 0.00 | 0.00 | 0.000 | 0.00–0.00 |
| Cautery, no ACT/APT | 0.46 | 0.19 | −1.89 | 0.059 | 0.21–1.03 |
| No cautery, yes ACT/APT | 0.33 | 0.14 | −2.57 | 0.010 | 0.14–0.77 |
| Cautery, yes ACT/APT | 0.49 | 0.23 | −1.50 | 0.134 | 0.19–1.25 |
| Reference: no packing | 1.00 | 0.00 | 0.00 | 0.000 | 0.00–0.00 |
| Both DP + NDP | 0.76 | 0.49 | −0.43 | 0.670 | 0.21–2.72 |
| DP only | 0.24 | 0.10 | −3.32 | 0.001 | 0.10–0.56 |
| NDP only | 0.61 | 0.27 | −1.12 | 0.261 | 0.26–1.45 |
| Received counselling | 0.92 | 0.27 | −0.29 | 0.768 | 0.51–1.65 |
| Received ointment | 0.62 | 0.18 | −1.62 | 0.106 | 0.35–1.11 |
| Age (years) | 1.00 | 0.01 | −0.09 | 0.930 | 0.98–1.02 |
| Hypertension | 0.98 | 0.22 | −0.07 | 0.942 | 0.64–1.51 |
| Arrhythmia | 0.99 | 0.33 | −0.04 | 0.969 | 0.51–1.90 |
| Constant | 7.30 | 3.99 | 3.63 | 0.000 | 2.50–21.33 |
| Number of observations | 335 | ||||
| LR chi2 | 31.73 | ||||
| Prob > chi2 | 0.0008 | ||||
| Pseudo R2 | 0.0791 | ||||
| Log likelihood | −184.8176 | ||||
Std. Err, standard error; CI, confidence interval; ACT, anticoagulation therapy; APT, antiplatelet therapy; DP, dissolvable packing; NDP, non-dissolvable packing; LR, logistic regression.
Looking at the long-term outcomes (>6 weeks), patients who were on ACT/APT had lowered odd of success. Cautery irrespective of the ACT/APT status had significantly worsen the outcome. Counselling and topical ointment application significantly increased odds of success. DP (Nasopore) worsens the odds short-term but there was no significant change long term (Table 4).
Table 4
| Treatment type | Odd ratio | Std. Err | z | P>z | 95% CI |
|---|---|---|---|---|---|
| Reference: no cautery or ACT | |||||
| Cautery, no ACT | 0.27 | 0.12 | −3.05 | 0.002 | 0.11−0.62 |
| No cautery, yes ACT | 0.25 | 0.11 | −3.03 | 0.002 | 0.10−0.61 |
| Cautery, yes ACT | 0.12 | 0.06 | −4.40 | 0.000 | 0.04−0.30 |
| Reference: no packing | |||||
| Both types | 0.39 | 0.26 | −1.42 | 0.155 | 0.11−1.43 |
| Dissolvable only | 0.52 | 0.25 | −1.36 | 0.173 | 0.20−1.33 |
| Non-dissolvable only | 0.72 | 0.36 | −0.65 | 0.513 | 0.28−1.90 |
| Received counselling | 3.38 | 1.21 | 3.41 | 0.001 | 1.68−6.82 |
| Received ointment | 3.63 | 1.35 | 3.47 | 0.001 | 1.75−7.50 |
| Age (years) | 1.01 | 0.01 | 0.81 | 0.418 | 0.99−1.03 |
| Hypertension | 0.86 | 0.18 | −0.70 | 0.483 | 0.57−1.31 |
| Arrhythmia | 0.82 | 0.28 | −0.56 | 0.574 | 0.42−1.62 |
| Constant | 3.75 | 2.27 | 2.19 | 0.029 | 1.15−12.26 |
| Number of odds | 335 | ||||
| LR chi2 | 59.24 | ||||
| Prob > chi2 | 0 | ||||
| Pseudo R2 | 0.1476 | ||||
| Log likelihood | −171.05914 | ||||
Std. Err, standard error; CI, confidence interval; ACT, anticoagulation therapy; APT, antiplatelet therapy; DP, dissolvable packing; NDP, non-dissolvable packing; LR, logistic regression.
The severity of the bleeding failed to show meaningful impact due to the small sample size. Majority of the cohort (91.9%) were discharged with 24 hours of admission. In the 27 cases (8.1%) that did not combination of patient’s comorbidities, COVID restrictions and unit logistics contributed to the increased LOS. 24.1% representing with recurrence within a year of admission.
Adverse events
Two patients required transfusion. Three patients died, one 51 years old and from ventricular fibrillation arrest following cessation of ACT (for known arrhythmia); and two (one frail) from sepsis secondary to aspiration pneumonia secondary to epistaxis. All deaths occurred in subsequent readmission to hospital within 14 days from discharge from hospital post management of epistaxis.
Discussion
There is evidence that certain individual treatment is associated with better outcome. However, the findings are not conclusive due to sample size, high variability of treatment combination, and lack of consistent guidelines for each subgroup of patients. This study and the literature review, identifies factors to consider for a well-designed large-scale controlled study to clarify the most cost-effective treatment for each subgroup of patients presenting with epistaxis.
Literatures reports, patients with epistaxis stay in hospital for 3 days on average; and over 16% are readmitted with recurrence within a year of admission (23,25). Despite the conservative management in this cohort, majority of the patients (91.9%) had a lower LOS (1 day), but a higher portion (24.1%) represented with recurrence within a year of admission.
Predictors of increase LOS as reported in literature are: APT/ACT (particularly combination), arterial HT, DM, posterior epistaxis, recurrent bleed, packing, electrocautery, or blood transfusion (23). The later three potentially reflect the severity of the bleed.
Predictors of readmission for recurrence are gender (male), DOAC, use of >1 ACT, LOS >3 days, recurrent bleed during admission, and nasal packing (23).
Posterior epistaxis was excluded in this study due to the small sample size. Electrocautery was not used in this cohort. The effect of the rest of the predictors of increased LOS and recurrence were analysed.
Anticoagulation
Various studies report increased incident of epistaxis with increased use of any ACT (26), but its impact on the degree of severity, and readmission rates or recurrence is not clear (23). This study found increased incidence of epistaxis and recurrence in ACT group. The impact on degree of severity was limited by the small sample size for major bleed.
Up to 68% of patients present with epistaxis are on long term ACT 85% have INR outside specified range, and 5.8% have pathological INR (26-28). Most common reason to be on ACT is AF. Often this group is older. In this study the findings were comparable to other literature, however subgroup analysis (Warfarin vs. DOAC) was limited by the small sample size in Warfarin group.
Those on Warfarin require a longer LOS (4 days) compared to non-ACT group (2 days). Bleeding risk and LOS exponentially increases with rising INR. One in 12 patients require an intervention. Adequate INR management can reduce the need for the interventions (7).
There is no evidence that DOAC increases risk of epistaxis, but patients are more likely have relapses (22,29). This could be the result of treatment choice rather than DAOC effect. Patients on DOAC also have significantly lower rate and severity of bleed some studies report higher rate of admission, transfusion, or intervention; that can reflect a more proactive management in the face of novel drugs (26,30). There are reports of DOAC epistaxis to be harder to control, but that can reflect the variability of care and lack of clear consensus on what treatment combination gives the best outcome (31,32).
Antiplatelet
APT are associated with increased frequency and severity of epistaxis (33); and higher risk for admission. Being on APT alone is not associated with increased rate of morbidity or complications, but if patient has more than one medical co-morbidity and on APT, it increases the risk of recurrence (within 28 days) (34). This study finds similar impact from APT.
Combined anticoagulation + antiplatelet
Combination medication increases frequency of recurrence (27% of cases) in comparison to ACT (Warfarin alone 9.4% or DOAC alone 14%) (26). This study also found significant increase recurrence with combination therapy.
Comorbidities
Seventy-seven percent of patients have one or more underlying disease. Comorbidities were not associated with severity of the bleed (P=0.2). The comorbidities that are associated with increased risk of bleeding were similar to the finding of this study but wider range including: arterial HT, nicotine or alcohol abuse, hypercholesterolaemia, AMI, coronary artery disease, stroke, chronic renal failure, and severe liver disease. Patients with DM or end-stage renal failure have disproportionate prolonged LOS (35).
HT
HT is the most common comorbidity in those presenting with epistaxis (28), and leads to increased risk of admission (26). Although 72.2% of patients meet the criteria for diagnosis (BP >140/90), only 33% to 50% have a known diagnosis of HT (27).
Up to 12.7% of patients present with hypertensive crisis (23).
HT is suspected as a casual factor in onset of epistaxis, but this is controversial (36). Various pathological mechanisms have been proposed including structural alteration to vessels due to raised arterial pressure (37). Detailed histological assessment shows reduction in elastic fibres due to collagen accumulation, with subsequent arterial stiffening and intima-media thickening that predisposes the vessels of nasal mucosa to rupture (36). Some also report HT increases risk of thrombosis and necrosis in the nasal mucosa, which further leads to bleeding (38).
The largest retrospective study (n=2,405) shows a small but significant increase in number of episodes of epistaxis in patients with HT (38). The metanalysis found a significant associated between HT and risk of epistaxis, but the association does not support a causal relationship (39). The population-based studies have revealed an association between epistaxis and HT, but population-based studies specific to epistaxis are rare (40).
Although the evidence to prove HT causes epistaxis is controversial, both the peripheral and CVD sequalae to HT, increases risk of epistaxis with odd ratios of 13.47 [95% confidence interval (CI), 1.90–95.28; P=0.009] and 3.91 (95% CI, 1.58–9.66; P=0.003) respectively (41).
Whilst elevated BP could be a result of anxiety associated with bleeding or the treatment (42), this study observes 121 episodes, where patient had high BP but no tachycardia, raising suspicion that the patient is not anxious but may have an unknown diagnosis of HT. Therefore, if all high BPs are not followed up with repeat BP monitoring to exclude presence of an unknown diagnosis of HT; the incidence of HT may be underestimated when assessing its impact on epistaxis incidence and recurrence. Looking at other literature, the studies largely fail to report if patients with high BP but no diagnosis of HT, were followed up to exclude presence of an unknown diagnosis of HT (43). Nevertheless, despite the controversy around the impact of HT on epistaxis, and limitation of reporting HT incidence, HT was found to be a significant variable in this cohort and therefore included in logistic regression.
Severe epistaxis is associated with underlying HT in 43% of the cases (level 2 evidence) (44). However, in this study only 3 cases out of 163 who had known HT, had severe epistaxis. This may be because most patient’s HT was well-controlled, and only 14 presented with hypertensive crisis (SBP >200). This was consistent with literature reporting the severity of controlled HT does not correlate with severity of epistaxis (level 2 evidence) (44).
Age
Mean age of adults presenting with epistaxis is 53.4. The incidence of epistaxis continuously increases for those older than 45 years with a steeper curve if older than 70 (28,37,45). Age is also associated with increased risk of severity, treatment failure and recurrence (26,29,46). Epistaxis burden also increases with clinical frailty score (>3) leading to increase rate and length of admission (11,47). In this cohort two of the deceased patients were elderly, one with a high frailty score (>3).
Impact of COVID
COVID testing increases rate of epistaxis (48-50). However, the sample size in this study was too small to see a significant increase.
There are no surgical procedures in this cohort. This may reflect the impact of COVID on management decisions (reduced instrumenting the airway when possible). The sample size was too small to confirm this with certainty.
The shift in management with COVID, reported to increase the duration of packing from 1.8 to 3 days (51) and was associated with increase recurrence (11% vs. 30%). These changes were not observed in this study, and may be due to the lower burden of COVID in Australia compared to the global experience.
Treatment types
Cessation of ACT/APT
Antiplatelets irreversibly inhibit platelet function for 5–7 days. Hence, stopping APT does not contribute to initial management of epistaxis including efficacy of packing; but can be considered in recurrent disease when the risk to interruption of APT is acceptable (36,52). Haemorrhage risks as not dose-dependent with aspirin monotherapy (49).
Factor Xa inhibitors not only have a shorter half-life, but also a wider therapeutic range, and a predictable pharmacokinetics and pharmacodynamics compared to Warfarin (50). Ideally patient on DOAC to stop DOAC 48–72 hours prior to any intervention, or 12–24 hours for emergency surgery (53). Antidotes are available but hard to access (22). However, it is well proven that epistaxis can be safely managed without stopping anticoagulation (54).
Ointment
There is a disparity of reports on effect of ointment. This could reflect variety of ointments including those containing irritants to the nasal mucosa. Lubrication particularly in setting of post cautery can avoid crusting and promote healing. Some studies report ointment outperforms cautery (in recurrent epistaxis) (55,56); but randomized controlled trials show no statistical significance in treatment outcomes. This study consolidates the contrast in outcomes by dividing the treatment outcome into short-term and long-term to demonstrate that ointment can reduce risk of recurrence long-term but does not change outcome short-term.
Cautery
Two types of cautery are frequently used: chemical and electrocautery. In this study no patients received electrocautery.
Chemical cautery is often the most common treatment modality for anterior epistaxis (4). Silver nitrate is readily available, safe, and easy to use (55,57).
Although various studies report cautery to be the most successful method for primary epistaxis (2,28), controlling up to 50% of epistaxis refractory to vasoconstrictors (28); this study found it significant reduced odds of success both short-term and long-term.
This could be due to various reasons such as the difference between the definition of failure in studies, the presence of electrocautery data which is superior to both chemical cautery and packing in controlling epistaxis (hence reducing both LOS and recurrence (level 2 evidence failure rates 12% vs. 22%) (49). Furthermore, the success of chemical cautery depends on identification, and accessibility of the offending vessel (4). Nasendoscope-assisted cautery has been shown to significantly reduce admission rate (in 74%), complications (0 vs. 44%) and recurrence (0 vs. 20%) (58). In this study no patient received nasendoscope.
Finally, not all patients bleed because of an offending vessel. There are other causes of bleeding such as mucosal tear or trauma to a dry mucosa, taking ACT/APT or mucosal irritation from an underlying disease. It was not possible to distinguish between the nature of bleed in retrospective study of the medical records.
Packing
Several types of packing are available, broadly divided into DP and NDP. Up to 92% of patients in non-tertiary centres are initially managed by NDP. Only 7–11% need intervention after arrival to the tertiary centre and removal of the packing (28); these are often patients with risk factors for relapse/recurrence (45).
Good results are reported if packing is applied for 1–3 days (4). However, NDP in return increases the LOS to 3.25 nights in average (34).
Packing is not harmless. It is associated with severe discomfort but also serious complications such as aspiration, cerebrospinal fluid (CSF) leak, pneumoencephalocoele (59-61).
NDP
Most of NDP used in this study was rapid rhino with exception of 9 patients who had Merocele.
Cost of packing with NDP is higher than chemical cautery, however studies reporting this are not controlled studies (25). Failure rate of NDP is also significantly higher than chemical cautery with an odd ratio of 6.08 (95% CI, 2.17–17.09); however, NDP is the most common intervention used for posterior epistaxis which has higher rate of relapse than anterior epistaxis (4).
The duration of packing also impacts outcome. The rate of rebleeding increases if NDP is applied shorter than 12 hours or longer than 24 hours (62-66).
DP
Nasopore was the DP of choice for all patients in this study. It was as readily available as rapid rhino to all medical practitioners.
DP is well known to provide excellent haemostasis post operatively with significant reduction in post operative pain, reduced nasolacrimal obstruction and epiphora from irritation (49). Expert opinion recommends these dressing in coagulopathy or patients on ACT/APT (49).
However, this study identified reduced odds of success in short term with DP. This could reflect higher rate of cautery (60%) prior use of DP, presence of comorbidity or ACT/APT (50%), all of which increased risk of relapse/recurrence.
Education
Patients who receive education on management of their epistaxis are less likely return to ED (67). Unfortunately, only 11% of public are aware of the correct area to apply pressure whilst bleeding. When they are educated, only 8% of the 17% recurrent epistaxis return to ED (68).
A study of 241 Junior doctors (JMO) revealed that up to 41% of JMOS are also unaware of the correct area to apply pressure. And only 18% were confident enough to perform nasal cautery which in turn increased LOS with patient awaiting transfer to a tertiary centre or review by ENT (69). Moreover, even if they are trained to manage epistaxis, the vital tools are often unavailable in many ED especially those without a provisional ENT trainee (up to 50%) (22,70). All these factors were impossible to identify in this retrospective case series.
Promising, all above measures improved with simple educational interventions implemented nationally in UK (71,72). This multidisciplinary approach reduced admission, days with nasal packing, cost and improves patient care (73).
Outcome
Length of admission
The only predictors of admission are old age, peripheral vascular diseases (PVD), CVD and previous history of epistaxis (41).
Admission may be an indication of either severity of epistaxis (clinical instability) or frailty of the patient (36), however this was poorly documented in medical records.
LOS was lower when patient received cautery vs. packing. However, if patient received a more invasive treatment (i.e., electrocautery with endoscope-assistance), they required significantly smaller number of interactions & had reduced LOS even in recurrent disease (4).
ACT patients had shorter LOS but more recurrence; but the recurrent episode would need less invasive therapy (hence reduced LOS) (26).
There was higher level of admission in remote or rural area attributed to lack of access to ENT surgeons (35).
Recurrence/readmission
13.9% of patients return with epistaxis within 30 days of initial episode (27,34,74); and 37% within a year but as high as 50% long term (75).
Many factors contribute to recurrent ED visits, many of which are independent risk factors for epistaxis: age older than 76, congestive cardiac failure [odds ratio (OR) =1.49], DM (OR =1.18), and OSA (OR =1.32) (41). HT, AF, PE, or mechanical heart valve did not affect readmission rates significantly (24,76).
Short-term and long-term relapses are higher in ACT group, especially if combined with APT. In contrast APT alone are not reported to be associated with recurrence (77), although they increased risk of admission or severity of bleed.
The treatment type also is a risk factor for recurrence. Packing/cautery group (OR 1.61) are significantly more likely to be admitted to ED within a year of their initial visit (24). Although the treatment choice can reflect the severity of the bleed and hence the recurrence; Kindler et al. suggests that it is the nasal crusting that attributes to the rate of post treatment complications (75).
Patients who receive more invasive treatment (all recurrent epistaxis) need less further attempts to achieve lasting haemostatic control. Direct electrocautery or proximal vascular control had significantly lower number of total required interventions, reduced LOS, and recurrence compared to less invasive methods (4). Surgical treatment has a 78% success in 7 years follow-up (75), and in the short term, is comparable to overall 20% recurrence rate for all treatment modalities. Finally routine systematic endoscopic assessment beyond Kiesselbach area for all patients reduce risk of recurrence. However, these studies include combination of both anterior and posterior epistaxis, and reserved intervention to recurrent cases. The risk of recurrence differs between patients with anterior vs. posterior epistaxis, and those presenting with recurrent epistaxis vs. primary episode.
Even though the initial cost of surgical invasive methods was high, its success in preventing return to hospital and lengthy admissions, can make them a cost-effective option.
Adverse events/complications
None of the treatment choices are risk free and associated with various adverse events ranging from mucosal irritation and scaring, to serious complications such as septal perforation or CSF leak, pneumonia (aspiration), and rarely death from bleeding, aspiration, or cessation of ACT/APT (11,59-61). Up to 23% receive blood transfusion, and 9.8% die of epistaxis or sequalae of disease or its treatment (74,78).
Most studies either do not report the mortality rate or underestimate the rate by focusing on short-term outcomes, or not looking for re-admissions under other specialities such as aspiration pneumonia within two weeks of epistaxis, under general medical bed card. Seeking otolaryngology input in such cases may identify further unidentified small source of bleed that can contribute to recurrent aspiration (either missed bleeding source or bleed from post cautery crusting or trauma from nasal packing). Further cohort studies with clear ENT input and detailed medical records would be helpful in identifying the true burden of epistaxis and its management especially in elderly and frail.
Limitations
Although the study was conducted in Australia’s largest multicentre tertiary hospital, the subgroup analysis of the data was limited in size for some patient or treatment groups due to large variability of treatment combination for each subgroup. Therefore, the findings require verification by a large-scale well-designed controlled study.
Overall conservative measures to treat epistaxis are very successful, and when the incidence of an outcome is high, the odds ratio may overestimate. This study tried to reduce this effect by using the odds ratio as the primary measure of the effect in multivariate logistic regression.
Retrospective case series could lead to recall bias. There were missing data on potential confounding factors (i.e., stage of liver disease or frailty) or contributing factors (i.e. platelet count). Although the exact degree of severity of bleed was not possible to identify, any other clues in medical records were utilised to broadly separate degree of bleeding to mild vs. severe.
Some treatment options (i.e., electrocautery, endoscopic assisted) was not used, hence could not be included in the comparative analysis. High blood pressures were not addressed (or if did, not documented) to assess the effects of the BP control (acutely or long term) in control of epistaxis and its recurrence.
The choice of treatment appeared to be dictated by the clinician’s routine preferred approach, but it also reflects severity of bleed. This was more challenged by the impact of COVID, as clinicians changed their routine practice to avoid instrumenting the airway. However, when bleeding was notably severe the treatment was escalated regardless of clinician’s preference or COVID restrictions.
Comparing the literature was challenging as the definitions of HT, recurrence, short- and long-term success varied in various studies.
Conclusions
Epistaxis is a multifactorial disease with high variability in treatment combination. There are over 5 clinical practice guidelines, 17 systematic reviews and 16 randomised clinical trials in English literature, however there is a lack of consensus on clear management algorithm for each subgroup of patients. Lack of standard approach makes further studies susceptible to bias with large heterogenous patient population receiving variety of treatment combinations.
Epistaxis treatment choice, itself can be an independent risk factor for recurrence of epistaxis, leading to prolonged admissions and further complication and rarely death. It is important to know which treatment would be the best choice when treating frail elderly patients with multiple comorbidities.
Epistaxis is a significant burden on healthcare resources. It is vital to reduce the recurrence of disease and length of stay by identifying the most cost-effective treatment combination for each subgroup of patients.
This study provides few factors to consider when designing a large-scale controlled study to further assess the impact of anticoagulation and choice of therapy on short-term and long-term outcomes.
Acknowledgments
This work was supported by department of research at Monash Health, Victoria, Australia, and with special contribution from Shrinkhala Dawadi, the mathematician who assisted with the analysis of the data.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-18/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-18/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-18/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure forms (available at https://www.theajo.com/article/view/10.21037/ajo-23-18/coif). ES serves as an unpaid editorial board member of the Australian Journal of Otolaryngology. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Monash Health (QA/69710/MonH-2021-263665). Because of the nature of this audit, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abdelkader M, Leong SC, White PS. Endoscopic control of the sphenopalatine artery for epistaxis: long-term results. J Laryngol Otol 2007;121:759-62. [Crossref] [PubMed]
- Shargorodsky J, Bleier BS, Holbrook EH, et al. Outcomes analysis in epistaxis management: development of a therapeutic algorithm. Otolaryngol Head Neck Surg 2013;149:390-8. [Crossref] [PubMed]
- Manes RP. Evaluating and managing the patient with nosebleeds. Med Clin North Am 2010;94:903-12. [Crossref] [PubMed]
- Morgan DJ, Kellerman R. Epistaxis: evaluation and treatment. Prim Care 2014;41:63-73. [Crossref] [PubMed]
- Ando Y, Iimura J, Arai S, et al. Risk factors for recurrent epistaxis: importance of initial treatment. Auris Nasus Larynx 2014;41:41-5. [Crossref] [PubMed]
- Badran K, Malik TH, Belloso A, et al. Randomized controlled trial comparing Merocel and RapidRhino packing in the management of anterior epistaxis. Clin Otolaryngol 2005;30:333-7. [Crossref] [PubMed]
- Murer K, Ahmad N, Roth BA, et al. THREAT helps to identify epistaxis patients requiring blood transfusions. J Otolaryngol Head Neck Surg 2013;42:4. [Crossref] [PubMed]
- Smith J, Siddiq S, Dyer C, et al. Epistaxis in patients taking oral anticoagulant and antiplatelet medication: prospective cohort study. J Laryngol Otol 2011;125:38-42. [Crossref] [PubMed]
- Lavy J. Epistaxis in anticoagulated patients: educating an at-risk population. Br J Haematol 1996;95:195-7. [Crossref] [PubMed]
- Spielmann PM, Barnes ML, White PS. Controversies in the specialist management of adult epistaxis: an evidence-based review. Clin Otolaryngol 2012;37:382-9. [Crossref] [PubMed]
- Stankovic P, Hoch S, Rudhart S, et al. Do all anticoagulants and antiplatelets really worsen the outcome of in-hospital epistaxis patients? Evidence from 447 Patients. Laryngorhinootologie 2022;101:243-4.
- Biggs TC, Baruah P, Mainwaring J, et al. Treatment algorithm for oral anticoagulant and antiplatelet therapy in epistaxis patients. J Laryngol Otol 2013;127:483-8. [Crossref] [PubMed]
- Mega JL, Simon T. Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet 2015;386:281-91. [Crossref] [PubMed]
- Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015;17:1467-507. [Crossref] [PubMed]
- Ng KP, Edwards NC, Lip GY, et al. Atrial fibrillation in CKD: balancing the risks and benefits of anticoagulation. Am J Kidney Dis 2013;62:615-32. [Crossref] [PubMed]
- Manchikanti L, Falco FJ, Benyamin RM, et al. Assessment of bleeding risk of interventional techniques: a best evidence synthesis of practice patterns and perioperative management of anticoagulant and antithrombotic therapy. Pain Physician 2013;16:SE261-318. [PubMed]
- Fenger-Eriksen C, Münster AM, Grove EL. New oral anticoagulants: clinical indications, monitoring and treatment of acute bleeding complications. Acta Anaesthesiol Scand 2014;58:651-9. [Crossref] [PubMed]
- Salem JE, Sabouret P, Funck-Brentano C, et al. Pharmacology and mechanisms of action of new oral anticoagulants. Fundam Clin Pharmacol 2015;29:10-20. [Crossref] [PubMed]
- Garcia-Callejo FJ. Effect of NOAC on non-hospital attendance. Annals of Otorrinolaringologia Mexico 2018;63:136-49.
- Tran H, Joseph J, Young L, et al. New oral anticoagulants: a practical guide on prescription, laboratory testing and peri-procedural/bleeding management. Australasian Society of Thrombosis and Haemostasis. Intern Med J 2014;44:525-36. [Crossref] [PubMed]
- García Callejo FJ, Bécares Martínez C, Calvo González J, et al. Epistaxis and dabigatran, a new non-vitamin K antagonist oral anticoagulant. Acta Otorrinolaringol Esp 2014;65:346-54. [Crossref] [PubMed]
- Ho JPK, Bari N, Riffat F. Management of epistaxis in patients on novel oral anticoagulation therapy. J Laryngol Otol 2020;134:316-22. [Crossref] [PubMed]
- Kallenbach M, Dittberner A, Boeger D, et al. Hospitalization for epistaxis: a population-based healthcare research study in Thuringia, Germany. Eur Arch Otorhinolaryngol 2020;277:1659-66. [Crossref] [PubMed]
- Chaaban MR, Zhang D, Resto V, et al. Factors influencing recurrent emergency department visits for epistaxis in the elderly. Auris Nasus Larynx 2018;45:760-4. [Crossref] [PubMed]
- Tathgar A, LeBihan G, Liu A. Managing epistaxis patients with nasal packs: Is it safe to discharge patients home with nasal packs in situ? Br J Surg 2020;107:86.
- Buchberger AMS, Baumann A, Johnson F, et al. The role of oral anticoagulants in epistaxis. Eur Arch Otorhinolaryngol 2018;275:2035-43. [Crossref] [PubMed]
- Tunkel DE, Anne S, Payne SC, et al. Clinical Practice Guideline: Nosebleed (Epistaxis) Executive Summary. Otolaryngol Head Neck Surg 2020;162:8-25. [Crossref] [PubMed]
- Richardson C, Abrol A, Hamill CS, et al. Improving efficiency in epistaxis transfers in a large health system: Analyzing emergency department treatment variability as pretext for a clinical care pathway. Am J Otolaryngol 2019;40:530-5. [Crossref] [PubMed]
- L'Huillier V, Badet C, Tavernier L. Epistaxis complicating treatment by anti-vitamin K and new oral anticoagulants. Eur Ann Otorhinolaryngol Head Neck Dis 2018;135:231-5. [Crossref] [PubMed]
- Send T, Bertlich M, Horlbeck F, et al. Management and outcome of epistaxis under direct oral anticoagulants: a comparison with warfarin. Int Forum Allergy Rhinol 2019;9:120-4. [Crossref] [PubMed]
- Carey B, Sheahan P. Aetiological profile and treatment outcomes of epistaxis at a major teaching hospital: a review of 721 cases. Ir J Med Sci 2018;187:761-6. [Crossref] [PubMed]
- Sauter TC, Hegazy K, Hautz WE, et al. Epistaxis in anticoagulated patients: Fewer hospital admissions and shorter hospital stays on rivaroxaban compared to phenprocoumon. Clin Otolaryngol 2018;43:103-8. [Crossref] [PubMed]
- Tay HL, Mcmahon AD, Evans JMM, et al. Aspirin, nonsteroidal anti-inflammatory drugs, and epistaxis. A regional record linkage case control study. Ann Otol Rhinol Laryngol 1998;107:671-4. [Crossref] [PubMed]
- Donaldson G, Goh KY, Tiwari P, et al. Anti-thrombotics and their impact on inpatient epistaxis management: a tertiary centre experience. Ir J Med Sci 2022;191:1621-9. [Crossref] [PubMed]
- Douglas CM, Tikka T, Broadbent B, et al. Patterns of hospital admission in 54 501 patients with epistaxis over a 20-year period in Scotland, UK. Clin Otolaryngol 2018;43:1465-70. [Crossref] [PubMed]
- André N, Klopp-Dutote N, Biet-Hornstein A, et al. Cardiovascular risk and severity factors in patients admitted to hospital for spontaneous epistaxis. Eur Ann Otorhinolaryngol Head Neck Dis 2018;135:119-22. [Crossref] [PubMed]
- Lee CJ, Seak CJ, Liao PC, et al. Evaluation of the Relationship Between Blood Pressure Control and Epistaxis Recurrence After Achieving Effective Hemostasis in the Emergency Department. J Acute Med 2020;10:27-39. [PubMed]
- Boiko NV. Structural changes in the nasal mucosa in the hypertensive patients suffering from recurrent epistaxes. Clin Rhinol 2018;112:44-8.
- Rezende GL, Oliveira LA, Soares RO, et al. Etiopathogenic features of severe epistaxis in histological samples from individuals with or without arterial hypertension. Sci Rep 2022;12:1361. [Crossref] [PubMed]
- Payne SC, Feldstein D, Anne S, et al. Hypertension and Epistaxis: Why Is There Limited Guidance in the Nosebleed Clinical Practice Guidelines? Otolaryngol Head Neck Surg 2020;162:33-4. [Crossref] [PubMed]
- Min HJ, Kang H, Choi GJ, et al. Association between Hypertension and Epistaxis: Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg 2017;157:921-7. [Crossref] [PubMed]
- Acar B, Yavuz B, Yıldız E, et al. A possible cause of epistaxis: increased masked hypertension prevalence in patients with epistaxis. Braz J Otorhinolaryngol 2017;83:45-9. [Crossref] [PubMed]
- Kim C, Kim C, Chung JH, et al. Is epistaxis associated with high blood pressure and hypertension? Propensity score matching study. Am J Emerg Med 2020;38:1319-21. [Crossref] [PubMed]
- Michel J, Prulière Escabasse V, Bequignon E, et al. Guidelines of the French Society of Otorhinolaryngology (SFORL). Epistaxis and high blood pressure. Eur Ann Otorhinolaryngol Head Neck Dis 2017;134:33-5. [Crossref] [PubMed]
- Nithianandan H, Thavorn K, Banaz F, et al. Determining the hospital cost of anterior epistaxis treatment modalities at a Canadian tertiary care centre. World J Otorhinolaryngol Head Neck Surg 2019;5:193-9. [Crossref] [PubMed]
- Côrte FC, Orfao T, Dias CC, et al. Risk factors for the occurrence of epistaxis: Prospective study. Auris Nasus Larynx 2018;45:471-5. [Crossref] [PubMed]
- Davies T, Alatsatianos A, Slim MAM, et al. The impact of frailty on epistaxis admission, a retrospective cohort study. Clin Otolaryngol 2021;46:983-90. [Crossref] [PubMed]
- Block-Wheeler NR, Wei J, Weintraub MR, et al. Providing Evidence for Dogma: Risk of Epistaxis After COVID-19 Nasal-Screening Swab. Otolaryngol Head Neck Surg 2023;169:47-54. [Crossref] [PubMed]
- Chandrasekar B, Oremule B, De Carpentier J. Epistaxis management in COVID-19. Br J Surg 2021;108:vi134. [Crossref]
- Laycook J, Ahmed O, Wasson J. Significant epistaxis secondary to COVID-19 nasopharyngeal swab. Br J Surg 2021;108:vi155.
- Admission avoidance in acute epistaxis: A prospective national audit during the initial peak of the COVID-19 pandemic. Clin Otolaryngol 2021;46:577-86. [Crossref] [PubMed]
- Rainsbury JW, Molony NC. Clopidogrel versus low-dose aspirin as risk factors for epistaxis. Clin Otolaryngol 2009;34:232-5. [Crossref] [PubMed]
- Bequignon E, Vérillaud B, Robard L, et al. Guidelines of the French Society of Otorhinolaryngology (SFORL). First-line treatment of epistaxis in adults. Eur Ann Otorhinolaryngol Head Neck Dis 2017;134:185-9. [Crossref] [PubMed]
- Bola S, Marsh R, Braggins S, et al. Does the continuation of warfarin change management outcomes in epistaxis patients? J Laryngol Otol 2016;130:256-60. [Crossref] [PubMed]
- Glikson E. In response to Epistaxis in the setting of antithrombotic therapy: A comparison between factor Xa inhibitors, warfarin, and antiplatelet agents. Laryngoscope 2019;129:E161. [Crossref] [PubMed]
- Ozmen S, Ozmen OA. Is local ointment or cauterization more effective in childhood recurrent epistaxis. Int J Pediatr Otorhinolaryngol 2012;76:783-6. [Crossref] [PubMed]
- Kumar S, Moorthy R. New oral anticoagulants - a guide for ENT surgeons. J Laryngol Otol 2016;130:324-8. [Crossref] [PubMed]
- Ha JF, Hodge JC, Lewis R. Comparison of nasendoscopic-assisted cautery versus packing for the treatment of epistaxis. ANZ J Surg 2011;81:336-9. [Crossref] [PubMed]
- Hollis GJ. Massive pneumocephalus following Merocel nasal tamponade for epistaxis. Acad Emerg Med 2000;7:1073-4. [Crossref] [PubMed]
- Sireci F, Immordino V, Galletti F, et al. Cerebrospinal Fluid Leak Following Nasal Packing for Epistaxis. J Craniofac Surg 2019;30:2536-8. [Crossref] [PubMed]
- Amin G, Rizvi ASA, Ahmad W, et al. Examine the impact of bilateral nasal packing on systemic blood pressure and prevalence of complication associated with nasal packing in patients who had undergone septoplasty. Pakistan Journal of Medical and Health Sciences 2020;14:1101-3.
- Abbas Y, Abdelkader M, Adams M, et al. Nasal Packs for Epistaxis: Predictors of Success. Clin Otolaryngol 2020;45:659-66. [Crossref] [PubMed]
- Collins R, Addison A, Paul C, et al. Nasal packing duration in the management of epistaxis: A cohort study. Br J Surg 2021;108:151. [Crossref]
- Walshe P. The use of fibrin glue to arrest epistaxis in the presence of a coagulopathy. Laryngoscope 2002;112:1126-8. [Crossref] [PubMed]
- Milinis K, Swords C, Hardman JC, et al. Dissolvable intranasal haemostatic agents for acute epistaxis: A systematic review and meta-analysis. Clin Otolaryngol 2021;46:485-93. [Crossref] [PubMed]
- Le A, Thavorn K, Lasso A, et al. Economic evaluation of floseal compared to nasal packing for the management of anterior epistaxis. Laryngoscope 2018;128:1778-82. [Crossref] [PubMed]
- Alexanderis-Souphis C, Haymart B, Felderisen D, et al. Reduction of ED visits for epistaxis in patients taking coumadin after a patient education program. J Thromb Thrombolysis 2019;47:614.
- Neshewat J, Wasserman A, Alexandris-Souphis C, et al. Reduction in epistaxis and emergency department visits in patients taking warfarin after implementation of an education program. Thromb Res 2021;199:119-22. [Crossref] [PubMed]
- Jamshaid S, Banhidy N, Ghedia R, et al. Where should epistaxis education be focused? A comparative study between the public and healthcare workers on knowledge of first aid management methods of epistaxis. J Laryngol Otol 2023;137:408-12. [Crossref] [PubMed]
- Fox R, Nash R, Liu ZW, et al. Epistaxis management: current understanding amongst junior doctors. J Laryngol Otol 2016;130:252-5. [Crossref] [PubMed]
- Banhidy N, Jamshaid S. Emergency medicine clinicians’ confidence in managing epistaxis is improved by a simple intervention: a closed-loop quality improvement study. Br J Surg 2021;108:vi148. [Crossref]
- Shaladi A, Loizou P, Michael M. The nosebleed effect: Advice given to epistaxis patients discharged from the emergency department. Br J Surg 2021;108:vi55. [Crossref]
- Vosler PS, Kass JI, Wang EW, et al. Successful Implementation of a Clinical Care Pathway for Management of Epistaxis at a Tertiary Care Center. Otolaryngol Head Neck Surg 2016;155:879-85. [Crossref] [PubMed]
- Williams R, Ellis M, Hall A, et al. Epistaxis 2016: national audit of management. J Laryngol Otol 2017;131:1131-41. [Crossref] [PubMed]
- Kindler RM, Holzmann D, Landis BN, et al. The high rate of long-term recurrences and sequelae after epistaxis treatment. Auris Nasus Larynx 2016;43:412-7. [Crossref] [PubMed]
- Jervis S, Saunders T, Belcher J, et al. Evaluating three hundred and fifty-two admissions and predictors of re-admissions for epistaxis - is it time to re-evaluate tranexamic acid in epistaxis? Clin Otolaryngol 2017;42:439-42. [Crossref] [PubMed]
- Cohen O, Shoffel-Havakuk H, Warman M, et al. Early and Late Recurrent Epistaxis Admissions: Patterns of Incidence and Risk Factors. Otolaryngol Head Neck Surg 2017;157:424-31. [Crossref] [PubMed]
- Corr MJ, Tikka T, Douglas CM, et al. One-year all-cause mortality for 338 patients admitted with epistaxis in a large tertiary ENT centre. J Laryngol Otol 2019;133:487-93. [Crossref] [PubMed]
Cite this article as: Ahmadi N, Sigston E. The impact of anticoagulation on management of anterior epistaxis: retrospective case series. Aust J Otolaryngol 2023;6:19.