
Evolution of neck dissections performed in conjunction with transoral robotic surgery lateral oropharyngectomy
Introduction
Oropharyngeal SCC accounts for 10% of the burden of head and neck squamous cell carcinoma, which is the 6th most common cancer worldwide (1). There is a rising incidence of disease in the oropharynx due to its causative relationship with the human papilloma virus (2). Since it was first described in 2005, transoral robotic surgery (TORS) has emerged as a safe and effective treatment modality for primary site disease (3-5). Surgical management of the neck is usually performed in conjunction with TORS.
TORS allows the surgeon 3D visualization and precise resection capabilities in the oropharynx. However, it is still susceptible to the complications seen in other forms of transoral surgery such as hemorrhage and orocervical communication (6). Haemorrhage after transoral surgery occurs in 3% to 10% of cases and is unpredictable and potentially catastrophic leading to mortality from aspiration and asphyxiation (7-9). Post-operative salivary leaks, while rarely associated with mortality, are a common cause of morbidity in transoral lateral oropharyngectomy when a neck dissection is performed concurrently, with rates of 1% to 15% (10,11).
A contentious proposition during TORS lateral oropharyngectomy and neck dissection is that ligation of the branches of the external carotid artery during neck dissection reduces the frequency of severe hemorrhage (12). Two studies have evaluated this hypothesis and both have found that there is no significant difference in bleeding rates between patients who undergo prophylactic ligation compared to those who do not undergo prophylactic ligation (13,14).
It has also been hypothesized that the timing of neck dissection could play a role in the prevention, or recognition and management, of post-operative orocervical leaks (15). Some centers suggest staging the neck dissection to give the primary site time to heal and therefore prevent orocervical communication, while other centers promote concurrent neck dissection and use of local, regional or free-tissue flaps as required to repair the observed intraoperative communication (16-19).
In our institution, preference has been for neck dissection to be performed concomitantly with the primary resection. However, to maximize utilization of limited TORS surgical lists there is potential advantage from performing a neck dissection prior to TORS lateral oropharyngectomy. The prophylactic ligation of the branches of the external carotid artery during the neck dissection can be performed to prevent hemorrhage and there is also an opportunity for pre-emptive reinforcement of the area at risk of orocervical communication to prevent leak, with a local muscle flap, further enhanced by the interval time that enables tissues to heal.
The purpose of this study was to evaluate the effect of neck dissection timing, and use of vessels ligation and muscle patch techniques, on secondary haemorrhage and orocervical communication rates in a small series at our institution.
Methods
Patient inclusion
The authors assert that all procedures contributing to this work comply with the ethical standards of the Royal Adelaide Hospital (RAH) Human Research Ethics Committee and with the Helsinki Declaration of 1975, as revised in 2013. A retrospective chart review of the RAH head and neck surgery department cancer database in combination with the hospital operative registry identified all patients who underwent primary TORS for tonsillar SCC with a neck dissection from August 2008 to October 2015. Patients were included if they had pathologically confirmed SCC located in the tonsil treated with TORS coupled with neck dissection. Exclusion criteria included other forms of trans-oral surgery such as transoral laser microsurgery, patients with non-SCC tumours and cancer in other sites of the oropharynx and head and neck. There were 33 patients who met the inclusion criteria. Of these patients there were 8 cases in the group who underwent neck dissection prior to TORS. There were 19 cases in which the neck dissection was performed concomitantly with TORS and 6 cases in which the neck dissection was staged following TORS.
Operative technique
After obtaining informed consent, all patients underwent TORS to resect their oropharyngeal squamous cell carcinoma using the da Vinci surgical robot (Intuitive Surgical Inc., Sunnyvale, CA, USA) via previously established resection techniques (3,20,21). Appropriate ipsilateral or bilateral neck dissections of levels I–V were either performed prior to, concomitantly with, or post- primary resection, as clinically indicated. The exact levels dissected were based primarily on N stage. Generally the affected nodal basin was dissected with a surrounding echelon of normal nodes. Bilateral neck dissections occurred on the rare occasion that the contralateral side was thought to harbor disease either radiologically or pathologically.
Vessel ligation
After completion of the neck dissection the external carotid artery was located and skeletonized. The facial, ascending pharyngeal and lingual arteries were identified and ligated with Weck metal ligation clips (Teleflex Inc., Research Triangle Park, NC, USA) (Figure 1).
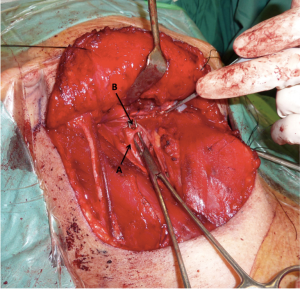
Prophylactic orocervical repair
After completion of the neck dissection and vessel ligation, attention was directed to the potential for orocervical communication secondary to TORS ablative surgery. The most common site is at the posterior aspect of the submandibular triangle, which lies adjacent to the floor of mouth and the tonsilo-lingual sulcus (Figure 2).
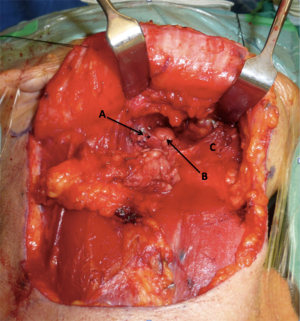
On the rare occasion of a demonstrable communication, the mucosa was directly repaired first. An anterior digastric muscle flap was used to reinforce the repair or, as in the majority of cases, pre-emptively reinforce this region. The muscle was mobilized anteriorly from its mandibular attachment and pedicled on the hyoid. The muscle was then sutured to the posterior edge of the mylohyoid muscle, periosteum of the mandible and to the posterior belly of the digastric muscle (Figure 3). Any primary mucosal repair, as well as the inset flap, was then reinforced with the fibrin sealant Tisseel (Baxter; Deerfield, IL, USA).
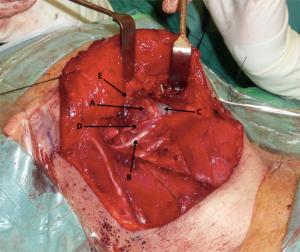
Data collection and analysis
An electronic spreadsheet was created to tabulate patient demographics, tumour location and staging, and timing of primary surgery and neck dissection. Data regarding the use of vessel ligation and fistula repair techniques and occurrence of hemorrhage and fistulae were entered into this spreadsheet. Data was retrieved from operative notes, pathology reports, hospital inpatient progress notes and outpatient department documentation.
Cases of post-TORS haemorrhage were classified according to a system for post-operative haemorrhage described by Pollei et al. (14). The tabulated data was analyzed with a specific focus on correlating hemorrhage and fistula rates with prophylactic surgical measures and timing of neck dissection.
Results
Patient demographics
Thirty-three consecutive patients who underwent TORS for tonsillar SCC coupled with neck dissection by the senior contributing authors were included in this retrospective analysis. In total 8 patients (24%) underwent neck dissection prior to TORS with a mean interval between operations of 8 days (Table 1). Nineteen patients (58%) had concurrent neck dissection with TORS, and 6 patients (18%) had their neck dissection following TORS with a mean interval between operations of 10 days.
Table 1
| Timing of ND | Cases [%] | Mean interval between operations (days) |
|---|---|---|
| Pre-TORS | 8 [24] | 8 |
| With TORS | 19 [58] | – |
| Post-TORS | 6 [18] | 10 |
TORS, transoral robotic surgery; ND, neck dissection.
The mean age of patients was 55 and 67% of patients were male and 33% female. The pathology confirmed T staging of the 33 patients as follows: 7 T1 (21%), 19 T2 (58%), 3 T3 (9%) and 4 T4a (12%). The N staging of the 33 patients was as follows: 7 N0 (21%), 1 N1 (3%), 3 N2 (9%), 5 N2a (15%), 16 N2b (48%), 0 N2c, and 1 N3 (3%). No patients had distant metastases. Two patients had stage II disease (6%), 4 patients had stage III disease (12%), and 27 patients had stage IV disease (81%).
Incidence of hemorrhage (Table 2)
Table 2
| Bleeding data | No vessel ligation documented | Vessels ligated pre-TORS | Vessels ligated with TORS | Vessels ligated post-TORS |
|---|---|---|---|---|
| Patients | 19 | 6 | 6 | 2 |
| Post-operative bleeds | 1 | 0 | 0 | 1 |
| Mean post-operative day of bleeding | 5 | – | – | 1 |
| Bleeds requiring operative intervention | 1 | – | – | 0 |
| Rate of bleeding | 5% | 0% | 0% | 50% |
TORS, transoral robotic surgery.
Of the 33 patients included in this study, 14 patients (42%) had documentation in their operative record of vessel ligation. Nineteen patients (58%) did not have documented vessel ligation. There was one case of major haemorrhage in this study, which required return to theatre, and this patient did not have branches of his external carotid artery ligated initially. None of the 14 patients who had vessel ligation experienced major postoperative haemorrhage.
Of the 14 patients who were documented to have had vessel ligation, 6 of these patients underwent neck dissection with vessel ligation prior to TORS, 6 patients had neck dissection and vessel ligation concurrently with TORS and 2 patients had neck dissection after TORS. One of the patients who was scheduled to undergo staged neck dissection following TORS had a mild tonsillar bleed on the first post-operative day of TORS, prior to his staged neck dissection, that resolved spontaneously and did not require return to theatre.
One patient out of the 33 (3%) included in this study had major haemorrhage, which required return to theatre. This was a 46-year-old male with a T2N2aM0 right tonsillar SCC. He had an ipsilateral level I–V neck dissection concurrently with the TORS resection of his tonsillar primary. He did not have the branches of his external carotid artery ligated during neck dissection. He was un-irradiated with no bleeding diathesis. On the fifth post-operative day he had a major haemorrhage from his right tonsillar bed and an urgent return to theatre was required. Topical measures were initially ineffective in haemostasis, so the neck was re-entered and selective arterial ligation performed. This resulted in haemostatic control. He recovered well from this episode with no sequelae or further secondary bleeds.
Incidence of orocervical communication (Table 3)
Table 3
| Intraoperative findings and repair procedure | Neck dissection preceding TORS | Neck dissection concurrent with TORS | Staged neck dissection following TORS |
|---|---|---|---|
| Intra-operative communication + repair (post-op leaks) | 0 (0) | 3 (0) | 2 (0) |
| Pre-emptive muscle patch (post-op leaks) | 4 (0) | 1 (0) | 1 (0) |
| No intra-operative communication, no pre-emptive muscle patch (post-op leaks) | 4 (0) | 15 [1] | 3 (0) |
TORS, transoral robotic surgery.
During each neck dissection, level Ib was assessed for the intra-operative presence of an orocervical communication. In six cases prophylactic reinforcement of these areas at risk of orocervical communication was performed. There were four cases of prophylactic repair of the potential area of orocervical communication in the neck dissection preceding TORS group, and one case each in the concurrent and post-TORS neck dissection groups. In all six of these instances there was no post-operative leak. Of the 8 patients who had neck dissection prior to TORS, there were no cases of post-operative orocervical communication and salivary leak. The 4 patients who did not have prophylactic orocervical communication repairs also did not develop a post-operative leak.
Of the 19 patients who underwent TORS and neck dissection concurrently, 3 patients had documented evidence of orocervical communication identified intraoperatively. In the group of patients who underwent TORS followed by neck dissection, 2 of 6 had documented evidence of orocervical communication identified intraoperatively. In both groups of patients, in all cases where intraoperative communication was observed and documented, repairs were performed and none of these patients went on to have a post-operative leak.
There was only one patient in the study who developed a post-operative leak. This was a 48-year-old man with a T1N2bM0 right tonsillar SCC who underwent a concurrent neck dissection and TORS. Interestingly this man was not identified as having an orocervical communication intraoperatively and no prophylactic repair was performed. The discovery of a leak was made in the immediate postoperative period when the wound site was observed to inflate and deflate with respiration. He was treated conservatively with low-pressure wall suction, appropriate dressing, intravenous antibiotics and delay of oral diet with exclusive nasoenteric feeds for alimentary support. A subsequent barium study performed 12 days postoperatively showed no evidence of extraluminal contrast material to suggest a leak, at which time all of the above interventions were ceased. The leak did not reoccur thereafter.
Discussion
There is currently no universally accepted guidelines or gold standard for timing of neck dissection in patients undergoing TORS lateral oropharyngectomy for the treatment of tonsil carcinoma. Concomitant primary resection and neck dissection would allow for selective arterial ligation and identification and repair of a level 1b orocervical communication, or prophylactic repair of this area, to prevent postoperative salivary fistulas. The patient needs only one general anaesthetic and there is an overall reduction in the total treatment package time and potential risk of tumour progression that could occur with delay in initiation of definitive treatment (22). Unfortunately, this is not always feasible, due to limited access to TORS operative lists and the increasing number of patients presenting with HPV-related tonsil cancers. Maximization of the TORS list for primary resections can occur by staging the neck dissections on these patients, either before or after the TORS primary resection. Performing the neck dissection before the primary resection facilitates pre-resection selective arterial ligation and prophylactic repair of level 1b (where orocervical communications occur), although strong evidence to show a resultant reduction in secondary haemorrhage and salivary fistula rates remains to be elucidated.
The Royal Adelaide Hospital began TORS protocol for the treatment of tonsillar SCC in August 2008. In this time the type and timing of the neck dissection has evolved as a result of a better understanding of the postoperative journey. There are now two major modifications to a standard neck dissection that we would routinely advocate. These are selective arterial ligation of the facial, lingual and ascending pharyngeal arteries, and local muscle flap reinforcement of level 1b (even in the absence of an identified orocervical communication). In this retrospective study there were no secondary bleeds or salivary leaks in the patients in which the neck dissections (and modifications) were conducted either on the same day or the week prior. To facilitate the improved morbidity that comes from these modifications the neck dissection needs to be performed either concomitantly or prior to the primary resection. This study, although limited by small numbers, confirmed the feasibility and safety of such a protocol.
The literature is scarce regarding prophylactic external carotid system vessel ligation during neck dissection and incidence of post-TORS lateral oropharyngectomy hemorrhage. To assess post oropharyngectomy haemorrhage rates and associated risk factors Pollei et al. conducted a retrospective chart review of 906 patients who underwent any type of transoral surgery for oropharyngeal carcinoma (14). Transcervical external carotid system vessel ligation was performed with the primary resection in 15.6% of patients. There was no overall difference in bleeding rate or severity of bleeding in patients who underwent ligation versus those who did not. There was a trend toward reduced post-oropharyngectomy bleeding severity with vessel ligation and so this group of authors recommended ligation for patients at higher risk of haemorrhage such as those with high T-stage tumours, primary tonsil tumours and patients undergoing revision surgery. Mandal et al. undertook a retrospective review of 224 consecutive patients who underwent TORS for any indication at a single institution and found 22 patients (9.82%) who had varying degrees of post-operative bleeding (13). The results of this demonstrated that prophylactic transcervical arterial ligation did not significantly decrease overall post-operative bleeding rates, however, like the previous study, there was a trend toward decreased haemorrhage severity.
Repanos et al. undertook a systematic review to assess how timing of neck dissection following transoral laser microsurgery in various head and neck subsites impacts upon patient outcomes, including its impact on post-operative haemorrhage rates. Fifteen studies in this review directly made mention of bleeding after primary resection, giving a rate of 5.3%, however there was a significant paucity of information on how timing of the neck dissection and use of external carotid system vessel ligation correlated with rate of post-operative haemorrhage (23). Crawford et al. contend in a review that ligating vessels in a pre-staged neck dissection may theoretically make the operative field less bloody during a delayed primary site ablation although clear evidence for this does not exist (12). Unfortunately, given the retrospective nature of the present study, this was not measured and analyzed.
Several authors have reported the role of primary closure of ablative defects after TORS pharyngectomy and concomitant neck dissection to decrease fistula rates (16-19). Genden et al. performed musculomucosal advancement flap pharyngoplasties in 25 patients (with a concomitant velopharyngoplasty in 6 of these patients) in a prospective non-randomised clinical trial (19). Also included in the study were 6 patients who underwent radial forearm free-flap reconstruction. No patients in this study developed neck infection or salivary fistula. Nam et al. described their method of closure of orocervical communication in 13 patients who underwent TORS for tonsillar SCC and where orocervical communication resulted during concomitant neck dissection (17). In all cases, their method of primary closure of the defect and reinforcement with muscle coverage was achievable with no cases of post-operative pharyngocutaneous fistula formation.
When the neck dissection occurs prior to the primary resection no orocervical communication will be apparent, as the oropharynx remains in situ, and therefore communication into the mouth is not possible due to the interposition of this tissue. We would however still advocate reinforcing the potential leak area with a local muscle flap, given the timing interval between the neck dissection and TORS procedure. This occurs no more than a week apart to reduce the overall treatment package time. This is unlikely sufficient time for the oropharyngeal defect to fully mucosalize and protect from any potential orocervical communication.
While our study focused on the incidence of pharyngocervical communication in patients where level I was routinely dissected, it is worth noting that there is no consensus in the literature regarding the best management of level I during neck dissection for oropharyngeal primaries (24). Some authors consider level II to IV dissection to be sufficient (25) whereas other authors recommend the removal of level I in clinically positive necks with tonsillar primaries (26,27). It has been our practice to remove level I regardless of nodal status in tonsillar primaries given the small but universally reported risk of <10% of level 1 metastasis from the tonsil (25-28). This practice is supported in the literature by other authors (29,30).
There is also no consensus in the literature regarding the best timing of neck dissection in the management of primary oropharyngeal SCC (23). Moore et al. conducted a retrospective chart review of 148 patients who underwent TORS and concurrent neck dissection for oropharyngeal neoplasia and identified 29% as having an orocervical communication intraoperatively, all of which were managed according to the authors’ described algorithm for treatment of pharyngocervical communication (31). Of these, 4% developed a subcutaneous pharyngeal fluid accumulation requiring post-operative management. These authors concluded that concurrent neck dissection at the time of TORS resection is safe and feasible and that while orocervical communication is common intraoperatively, persistent fistula formation is uncommon and preventable with prompt recognition and intervention. Möckelmann et al. performed a prospective case series comparing 21 patients who underwent TORS and concurrent neck dissection with 20 patients who underwent neck dissection staged after their TORS resection (15). These authors reported no significant difference in complication rates between the two groups and recommended that staged neck dissection following primary resection is safe if there are theatre constraints that do not allow for concurrent resection. We would however advocate staging the neck dissection before the TORS resection.
Limitations of our study are its retrospective nature and the small number of patients included in this series, which diminishes its statistical power. Risk factors for post-operative haemorrhage and orocervical fistula have not been controlled for. Practical and economic factors associated with timing of neck dissection were not evaluated and questions surrounding the oncological and mortality outcomes relating to timing of resection of the primary site or the cervical lymph nodes remain unanswered.
In conclusion it would seem ideal that neck dissection and its modifications occur concurrently with the TORS resection, however where TORS operative time is limited then this can occur prior to the resection in a way that is safe and feasible. It does not compromise patient care and allows maximization of robotic theatre utilization. Pre-staged neck dissection prior to TORS provides the opportunity to ligate the branches of the external carotid artery and reinforce the areas at risk of orocervical communication as outlined in this paper. While the numbers in this series are small, this study adds to the scarce data in the literature on the effect of vessel ligation and orocervical communication repair techniques on post-operative haemorrhage and post-operative leak rates respectively. Further large-scale prospective clinical trials are necessary to define the precise value of these techniques and until such studies are complete, an expert consensus among highly experienced transoral head and neck surgeons may be helpful to guide standardization of such approaches.
Acknowledgments
This manuscript was presented at the Australian Society of Otolaryngology, Head and Neck Surgery (ASOHNS) Annual Scientific Meeting held in Melbourne, Victoria, Australia from the 6th–8th March 2016.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.01.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors assert that all procedures contributing to this work comply with the ethical standards of the Royal Adelaide Hospital (RAH) Human Research Ethics Committee and with the Helsinki Declaration of 1975, as revised in 2013. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kelly K, Johnson-Obaseki S, Lumingu J, et al. Oncologic, functional and surgical outcomes of primary Transoral Robotic Surgery for early squamous cell cancer of the oropharynx: a systematic review. Oral Oncol 2014;50:696-703. [Crossref] [PubMed]
- Ernster JA, Sciotto CG, O'Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope 2007;117:2115-28. [Crossref] [PubMed]
- Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope 2009;119:2156-64. [Crossref] [PubMed]
- Weinstein GS, O'Malley BW Jr, Magnuson JS, et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 2012;122:1701-7. [Crossref] [PubMed]
- O'Malley BW Jr, Weinstein GS, Snyder W, et al. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 2006;116:1465-72. [Crossref] [PubMed]
- Chia SH, Gross ND, Richmon JD. Surgeon experience and complications with Transoral Robotic Surgery (TORS). Otolaryngol Head Neck Surg 2013;149:885-92. [Crossref] [PubMed]
- Salassa JR, Hinni ML, Grant DG, et al. Postoperative bleeding in transoral laser microsurgery for upper aerodigestive tract tumors. Otolaryngol Head Neck Surg 2008;139:453-9. [Crossref] [PubMed]
- Tami TA, Parker GS, Taylor RE. Post-tonsillectomy bleeding: an evaluation of risk factors. Laryngoscope 1987;97:1307-11. [Crossref] [PubMed]
- Sarny S, Ossimitz G, Habermann W, et al. Hemorrhage following tonsil surgery: a multicenter prospective study. Laryngoscope 2011;121:2553-60. [Crossref] [PubMed]
- Henstrom DK, Moore EJ, Olsen KD, et al. Transoral resection for squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg 2009;135:1231-8. [Crossref] [PubMed]
- Rich JT, Milov S, Lewis JS Jr, et al. Transoral laser microsurgery (TLM) +/- adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope 2009;119:1709-19. [Crossref] [PubMed]
- Crawford JA, Bahgat AY, White HN, et al. Hemostatic Options for Transoral Robotic Surgery of the Pharynx and Base of Tongue. Otolaryngol Clin North Am 2016;49:715-25. [Crossref] [PubMed]
- Mandal R, Duvvuri U, Ferris RL, et al. Analysis of post-transoral robotic-assisted surgery hemorrhage: Frequency, outcomes, and prevention. Head Neck 2016;38:E776-82. [Crossref] [PubMed]
- Pollei TR, Hinni ML, Moore EJ, et al. Analysis of postoperative bleeding and risk factors in transoral surgery of the oropharynx. JAMA Otolaryngol Head Neck Surg 2013;139:1212-8. [Crossref] [PubMed]
- Möckelmann N, Busch CJ, Munscher A, et al. Timing of neck dissection in patients undergoing transoral robotic surgery for head and neck cancer. Eur J Surg Oncol 2015;41:773-8. [Crossref] [PubMed]
- de Almeida JR, Park RC, Villanueva NL, et al. Reconstructive algorithm and classification system for transoral oropharyngeal defects. Head Neck 2014;36:934-41. [Crossref] [PubMed]
- Nam IC, Park JO, Joo YH, et al. Role of primary closure after transoral robotic surgery for tonsillar cancer. Auris Nasus Larynx 2015;42:43-8. [Crossref] [PubMed]
- Howard BE, Hinni ML, Nagel TH, et al. Submandibular gland preservation during concurrent neck dissection and transoral surgery for oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg 2014;150:587-93. [Crossref] [PubMed]
- Genden EM, Park R, Smith C, et al. The role of reconstruction for transoral robotic pharyngectomy and concomitant neck dissection. Arch Otolaryngol Head Neck Surg 2011;137:151-6. [Crossref] [PubMed]
- Weinstein GS, O'Malley BW Jr, Snyder W, et al. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg 2007;133:1220-6. [Crossref] [PubMed]
- Holsinger FC, McWhorter AJ, Menard M, et al. Transoral lateral oropharyngectomy for squamous cell carcinoma of the tonsillar region: I. Technique, complications, and functional results. Arch Otolaryngol Head Neck Surg 2005;131:583-91. [Crossref] [PubMed]
- Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol 2007;84:5-10. [Crossref] [PubMed]
- Repanos C, Mirza AH, George M, et al. Timing of neck dissection in association with transoral surgery: A systematic review. Head Neck 2017;39:1020-32. [Crossref] [PubMed]
- Noel CW, Foreman A, Goldstein DP, et al. Extent of neck dissection after transoral robotic surgical resection of oropharyngeal squamous cell carcinoma: Report of a case and potential indications for inclusion of level I in a selective neck dissection. Head Neck 2015;37:E130-3. [Crossref] [PubMed]
- Li XM, Wei WI, Guo XF, et al. Cervical lymph node metastatic patterns of squamous carcinomas in the upper aerodigestive tract. J Laryngol Otol 1996;110:937-41. [Crossref] [PubMed]
- Wiegand S, Esters J, Muller HH, et al. Relevance of oropharyngeal cancer lymph node metastases in the submandibular triangle and the posterior triangle apex. Anticancer Res 2009;29:4785-90. [PubMed]
- Lim YC, Koo BS, Lee JS, et al. Distributions of cervical lymph node metastases in oropharyngeal carcinoma: therapeutic implications for the N0 neck. Laryngoscope 2006;116:1148-52. [Crossref] [PubMed]
- Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg 1990;160:405-9. [Crossref] [PubMed]
- Jose J, Coatesworth AP, Johnston C, et al. Cervical node metastases in oropharyngeal squamous cell carcinoma: prospective analysis of prevalence and distribution. J Laryngol Otol 2002;116:925-8. [Crossref] [PubMed]
- Vartanian JG, Pontes E, Agra IM, et al. Distribution of metastatic lymph nodes in oropharyngeal carcinoma and its implications for the elective treatment of the neck. Arch Otolaryngol Head Neck Surg 2003;129:729-32. [Crossref] [PubMed]
- Moore EJ, Olsen KD, Martin EJ. Concurrent neck dissection and transoral robotic surgery. Laryngoscope 2011;121:541-4. [Crossref] [PubMed]
Cite this article as: Krishnan G, David R, Gouzos M, Foreman A, Krishnan S, Hodge JC. Evolution of neck dissections performed in conjunction with transoral robotic surgery lateral oropharyngectomy. Aust J Otolaryngol 2018;1:6.


