Gastrostomy tube insertion outcomes in South Australian head and neck cancer patients
Introduction
Head and neck cancer is the 6th most common malignancy world-wide, affecting 600,000 patients annually (1). Despite advances in treatment modality, dysphagia is still a common post-treatment morbidity affecting greater than 50% of cancer survivors (2). Gastrostomy tubes are important adjuncts in maintaining nutrition in patients undergoing treatment for their head and neck cancer and sometimes are a permanent necessity for those suffering from severe long-term dysphagia after treatment of their cancer. There is currently no consensus regarding the role of gastrostomy tube insertions for head and neck cancer patients (3).
Treatment options for head and neck cancer currently include surgery, radiotherapy and chemotherapy alone or in combination. However, despite a general trend towards organ-preservation chemo-radiotherapy protocols, dysphagia is still common as it is recognised tissue preservation does not always equal function preservation (4). Dysphagia following non-surgical therapy is usually secondary to mucositis in the acute period and pharyngeal submucosal and muscular fibrosis in the long-term (5-7). These treatments associated side effects lead to hospital readmissions, malnutrition and disruption or delay in treatment.
Gastrostomy tubes can be inserted endoscopically (PEG) or radiologically (RIG) depending on patient factors, institution preference and access to expertise with gastrostomy tubes. Despite improvements in nutritional status of patients following gastrostomy tube insertions, potential complications including stomal site infections, bleeding, tube dislodgement and peritonitis. Thus, the risks associated with gastrostomy insertion must be weighed up against the risks associated with malnutrition and clearly discussed with the patient (8).
This study aims to investigate the nutritional status, readmission rates, gastrostomy dependency and complication rates in patients managed with gastrostomy insertions in two tertiary head and neck cancer centres in South Australia.
Methods
A retrospective chart review was performed on all patients with head and neck cancer treated with non-surgical modality at Flinders Medical Centre and Royal Adelaide Hospital from January 1st 2010 to December 31st 2015 with at least 12 months follow-up data. Inclusion criteria included patients greater than 18 years of age with a histological diagnosis of mucosal squamous cell carcinoma (SCC) in any head and neck subsite treated with primary radiotherapy or chemoradiotherapy aimed at curative intent. Patients managed with palliative intent were excluded.
Prior to treatment, patients were examined clinically and with a panendoscopy under general anaesthesia. Radiological examination consisted of computed tomography (CT) neck and chest and positron emission tomography (PET) scans where indicated. Tumour staging was based on the TNM classification of International Union Against Cancer (UICC) 7th edition (9). All cases were discussed in a multidisciplinary meeting where a decision was made regarding treatment modality. Decision on gastrostomy tube insertion was based on institution preference and pre-defined criteria (Table 1). Prophylactic gastrostomy insertion was defined by the multidisciplinary team (MDT) decision to insert the tube prior to treatment, whereas reactive tube insertion was defined as gastrostomies inserted after initiation of treatment.
Table 1
| T4 or hypopharynx tumours planned for chemoradiotherapy |
| T3 tumour of the oral cavity |
| T3 and/or N2 or N3 tumour of the oropharynx or larynx |
| Bilateral radiotherapy |
| Major surgery (major flap reconstruction, total glossectomy, or total laryngectomy) |
| Pre-operative radiotherapy followed by reconstruction with flap, particularly in oropharyngeal area |
| Significant dysphagia at presentation |
| Severe malnutrition at presentation |
| Unintentional weight loss >10% in 6 months |
| BMI <18.5 or BMI <20 (65 years or older) with unintentional weight loss of 5–10% in 6 months |
| PG-SGA—C |
BMI, body mass index; PG-SGA, patient-generated subjective global assessment.
The Human Research Ethics Committee at Flinders Medical Centre and Royal Adelaide Hospital granted ethics approval. All patients were identified through the electronic theatre management system (ORMIS) with a search query consisting of “percutaneous endoscopic gastrostomy”, “radiologically inserted gastrostomy”, “PEG” and “RIG” conducted between 2010 and 2015.
Medical records for all identified patients were reviewed to collect patient demographic details, clinical, radiology, pathology and treatment data. Patient demographics collected included age, gender, malignancy stage, completion of treatment, pre-treatment weight, post-treatment weight, duration of gastrostomy, complications from gastrostomy and readmissions. The aim of this study was to review the outcomes in patients treated with gastrostomy tubes. The primary outcomes were complication rates. These were grouped by gastrostomy insertion technique and stratified according to the Clavien-Dindo classification (Table 2) (10). Secondary outcomes included interruption to treatment, readmission rates, weight changes and gastrostomy dependency. Readmissions were stratified into readmissions to hospital within one year following treatment, 30-days following gastrostomy insertion or during active treatment. Weight loss defined as decrease in post-treatment weight compared to pre-treatment weight. All readmissions were within one year following treatment.
Table 2
| Grade I | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic and radiological interventions; acceptable therapeutic regimens are: drugs as antiemetics, antipyretics, analgesics, diuretics and electrolytes and physiotherapy |
| Grade II | Requiring pharmacological treatment with drugs other than such allowed for grade I complications; blood transfusions and total parenteral nutrition are also included |
| Grade III | Requiring surgical, endoscopic or radiological intervention |
| Grade IIIa | Intervention not under general anaesthesia |
| Grade IIIb | Intervention under general anaesthesia |
| Grade IV | Life-threatening complication (including CNS complications) requiring ICU management |
| Grade IVa | Single organ dysfunction |
| Grade IVb | Multi organ dysfunction |
| Grade V | Death |
CNS, central nervous system; ICU, intensive care unit.
Statistical analysis
Statistical analysis was performed utilizing the SPSS version 24. Parametric statistical analysis was performed (chi-squared tests, Fisher’s exact test) comparing patient demographic and complications between the PEG and RIG groups. As there was only one patient treated with surgical gastrostomy, they were excluded from the statistical analysis. Repeated measures ANOVA was used to investigate the changes in weight between time points. Spearman’s rho correlation analysis was conducted to find associations between repeated measures weights and gastrostomy dependence.
Results
Patient demographics
A total of 103 patients were identified as having received gastrostomy tube insertions over a 5-year period; 54 (52%) patients had a percutaneous approach, 48 (47%) patients had a radiological approach (Table 3). One patient with a T4bN0M0 hypopharynx SCC underwent a surgical gastrostomy. The majority of patients had prophylactic gastrostomy insertions compared to reactive insertions, 88 (85%) and 15 (15%), respectively. PEG’s were commonly employed in patients with prophylactic gastrostomy tubes, whereas RIG’s were utilized more commonly in patients with reactive gastrostomy tubes. The oropharynx was the most commonly involved subsite, consisting of 77 (75%) patients. All patients with T1 disease had at least N2a disease with overall stage 4a. No patients had delay in treatment secondary to malnutrition.
Table 3
| Variable | PEG | RIG | P value |
|---|---|---|---|
| Mean age | 58±10 SD | 58±11 SD | |
| Gender | >0.05 | ||
| Female | 9 | 10 | |
| Male | 45 | 38 | |
| Primary site | 0.048 | ||
| Nasopharynx | 1 | 3 | |
| Oral cavity | 2 | 2 | |
| Oropharynx | 46 | 31 | |
| Supraglottis | 0 | 1 | |
| Hypopharynx | 1 | 6 | |
| Larynx | 2 | 5 | |
| Unknown | 2 | 0 | |
| T-stage | 0.004 | ||
| x | 2 | 0 | |
| 1 | 5 | 5 | |
| 2 | 19 | 5 | |
| 3 | 11 | 7 | |
| 4a | 16 | 23 | |
| 4b | 1 | 8 | |
| N-stage | >0.05 | ||
| 0 | 11 | 12 | |
| 1 | 4 | 8 | |
| 2a | 8 | 3 | |
| 2b | 21 | 15 | |
| 2c | 9 | 8 | |
| 3 | 1 | 2 | |
| M-stage | >0.05 | ||
| 0 | 54 | 48 | |
| 1 | 0 | 0 | |
| Overall staging | >0.05 | ||
| 1 or 2 | 2 | 2 | |
| 3 | 7 | 6 | |
| 4 | 45 | 40 | |
| HPV status | 0.039 | ||
| Positive | 31 | 16 | |
| Negative | 7 | 13 | |
| Unknown | 16 | 19 |
PEG, percutaneous endoscopic gastrostomy; RIG, radiologically inserted gastrostomy; SD, standard deviation.
Gastrostomy complications
A total of 59 complications occurred in 44 (42%) patients following their gastrostomy tube insertions, of which 17 (16%) had PEG’s, 26 (26%) had RIG’s and 1 (1%) had surgical gastrostomy insertion. Four PEG patients and two RIG patients suffered two or more complications. The majority of the complications were minor complications such as excessive granulation tissue around the tube site or superficial skin infections (Table 4). This study found that grade IIIb complications all occurred in the RIG group, requiring surgical intervention under general anaesthetic for the treatment of peritonitis, intra-abdominal bleed and gastrostomy site fistula (Table 5). No patients died from gastrostomy complications during this study period. Total complications and complications stratified by grade were found to be statistically significant (P=0.027 and P=0.042, respectively). However, there was insufficient data to perform statistical testing for individual complications and grades.
Table 4
| Complication | PEG (%) | RIG (%) | P value |
|---|---|---|---|
| Overgranulation | 17 [53] | 2 [7] | >0.05 |
| Superficial Infection | 7 [22] | 10 [37] | |
| Bleeding | 1 [3] | 0 [0] | |
| Leaking | 1 [3] | 7 [26] | |
| Dislodged | 6 [19] | 4 [15] | |
| Peritonitis | 0 [0] | 1 [4] | |
| Fistula | 0 [0] | 1 [4] | |
| Balloon rupture | 0 [0] | 1 [4] | |
| Intra-abdominal bleed | 0 [0] | 1 [4] | |
| Total | 32 [100] | 27 [100] | 0.027 |
PEG, percutaneous endoscopic gastrostomy; RIG, radiologically inserted gastrostomy.
Table 5
| Grade | PEG (%) | RIG (%) | P value |
|---|---|---|---|
| No complications | 37 | 22 | 0.042 |
| I | 0 [0] | 0 [0] | |
| II | 25 [78] | 12 [44] | |
| IIIa | 7 [22] | 11 [41] | |
| IIIb | 0 [0] | 4 [15] | |
| IVa | 0 [0] | 0 [0] | |
| IVb | 0 [0] | 0 [0] | |
| V | 0 [0] | 0 [0] |
PEG, percutaneous endoscopic gastrostomy; RIG, radiologically inserted gastrostomy.
Readmissions
A total of 121 readmissions occurred in 73 (70%) patients, of which 36 (35%) patients had multiple re-admissions within one year of treatment (Table 6). And 35 (29%) admissions occurred within 30-days of gastrostomy insertion and 57 (47%) admissions occurred during treatment. Mucositis and acute pain requiring supportive care were the most common indications for readmission, accounting for 27 (22%) and 20 (17%) re-admissions respectively. Gastrostomy-related readmissions occurred in 18 (15%) patients.
Table 6
| Readmission indication | Re-admission (%) |
|---|---|
| Acute airway emergency | 7 [6] |
| Primary site bleed | 8 [7] |
| Mucositis | 27 [22] |
| Febrile illness | 16 [13] |
| Acute pain control requiring supportive care | 20 [17] |
| Gastrostomy complication | 18 [15] |
| Cardiac (APO/MI/AF) | 2 [2] |
| Respiratory (COPD/LRTI/pneumothorax) | 8 [7] |
| Gastrointestinal (SBO) | 1 [1] |
| Urinary retention | 1 [1] |
| Chronic pain | 13 [11] |
APO, acute pulmonary oedema; MI, myocardial infarction; AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; LRTI, lower respiratory tract infection; SBO, small bowel obstruction.
Weight changes between prophylactic and reactive gastrostomy
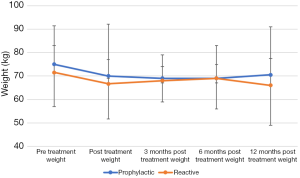
The prophylactic gastrostomy group consisted of 88 patients with a median pre-treatment weight of 75 kg (range: 41–133). The reactive gastrostomy group included 15 patients with a median pre-treatment weight of 71 kg (range: 36–93). Immediately post treatment, patients in both groups were found to have a median weight loss of 5 kg. The weight remained stable between immediate post-treatment and 12 months post treatment for both groups. Pre-treatment weights were found to correlate with the different time points (Figure 1, P<0.05). There was no correlation with weights to duration of gastrostomy in situ.
Gastrostomy tube removal
Prophylactic gastrostomy tubes remained in situ for a median 174 days (SD 292.4) compared to reactive gastrostomy tubes which remained in situ for median of 249 days (SD 204.8). There was no statistically significant difference due to the small group of reactive gastrostomy patients (P=0.41).
Discussion
This multi-institutional retrospective chart review examines the use of gastrostomy tubes in 103 head and neck cancer patients treated with primary non-surgical therapy in two South Australian tertiary head and neck centres. The use of gastrostomy tubes in the maintenance of nutrition in head and neck cancer patients during treatment is a controversial topic. Current literature demonstrates conflicting results with different institutions adopting varying protocols and treatment regimes (3,11,12). The decision for prophylactic insertion of a gastrostomy tube was made at our multi-disciplinary meetings, based on patient and treatment factors, before the patient began treatment of their cancer. Despite these issues, there was found to be no delay in treatment.
This study demonstrated overall complication rates for PEG and RIG insertions were 16% and 26% respectively. RIG insertions were found to have a greater number of grade III complications compared to the PEG group. Grade II complications occurred in 24% of patients, requiring pharmacological intervention. Grade III complications occurred in 18% of patients requiring surgical management. Brown et al. demonstrated minor and major complication rates of 40% and 10% respectively in Australian head and neck cancer patients (13). It was difficult to compare these results due to differing classifications of complications and 93% of Brown’s patients having PEG insertions. This study had comparable results to the systematic review conducted by Grant et al. demonstrating PEG and RIG complication rates of 19% and 24% respectively (8). Although no mortalities were present in this series, the current literature demonstrates fatality rates following PEG and RIG insertions of 2.2% and 1.8% respectively (8). Our results demonstrate lower rates of overall and serious complications with PEG’s. The choice of employing a PEG or RIG is dependent on patient factors, such as oral access (mouth opening, obstructive aerodigestive tract mass), previous abdominal surgeries, co-morbidities, institution and operator expertise. These results bring into question the use of nasogastric tube feeding as a safer alternative for short-term nutrition. Systematic reviews by Wang et al. and Paleri et al. demonstrated equivalent weight maintenance between the PEG’s and nasogastric feeding (14,15). Additionally, PEG’s were associated with increased incidence of pain and delay in return to oral diet compared to nasogastric tubes. Alternatively, patients with nasogastric feeds experienced altered body image with increased inconvenience compared to their PEG counterparts.
Readmissions to hospital were found to be due to acute pain management requiring supportive care secondary to mucositis. Mucositis affects up to 60% of patients receiving radiation therapy to the head and neck region, with a significant rise in incidence to greater than 90% when utilized on conjunction with chemotherapy (5). The significant pain associated with mucositis commonly leads to severe dehydration and malnutrition associated with hospital readmissions. Capuano et al. found reduced rates of hospital readmissions with stable maintenance of weight in patients with prophylactic gastrostomy insertions (16). Patients with prophylactic gastrostomies readmitted with dehydration could be due to non-compliance with gastrostomy use. Patient education and motivation on gastrostomy use must be considered prior to insertion; however, this was outside the scope of this study.
Maintenance of weight and nutrition is an important aspect in the treatment of patients with head and neck cancer. Patients treated in both prophylactic and reactive gastrostomy groups were found to lose a median weight of 5 kg during treatment, which stabilised by 12 months following treatment. These findings are similar to the study by Kramer et al. demonstrating no significant difference in rates of weight loss between patients with prophylactic or reactive gastrostomy tubes (11). Additionally, our study identified that pre-treatment weight was strongly correlated with post-treatment weight at all time points, demonstrating the importance of pre-treatment nutritional status on weight maintenance. These findings demonstrate the importance of optimising head and neck patient nutritional status prior to treatment. Capuano et al. and Paccagnella et al. demonstrated early nutritional intervention to be associated with improved weight maintenance, decreased infections, early mortality, survival, treatment tolerance and decreased readmissions (16,17). Isenring et al. demonstrated early nutritional supplementation resulted in improvement with global quality of life and physical function with reduction in treatment-associated morbidity (18).
Prophylactic gastrostomy tubes were found to be in situ in the patient for a shorter period compared to reactive gastrostomy tubes, being 174 and 249 days respectively. Brown et al. demonstrated median time to tube removal in prophylactic gastrostomy insertions ranging from 100 to 110 days (13). Additionally, they found 87% of patients were completely dependent on enteric feeds for their full nutritional needs at the completion of treatment. This rate dropped to 66% and 29% at one- and three-months post-treatment. The extend of gastrostomy usage was outside the scope of this study. The prolonged gastrostomy dependence present in the reactive group may demonstrate progressive swallowing impairment secondary to treatment with poor nutritional status. This group of patients may have had impaired swallowing prior to treatment predisposing them to increased incidence of failing oral feeding during treatment compared to patients completing chemoradiotherapy without gastrostomy feeds, this was outside the scope of this study. The increased gastrostomy dependence in these patients is likely secondary to the effects of chemoradiotherapy such as late oesophageal strictures, muscular fibrosis and disuse atrophy of pharyngeal muscles (12,19,20). Additionally, prolonged gastrostomy dependency has been associated with adverse effects on quality of life (15). The shorter gastrostomy dependency within the prophylactic group could be due to that group of patients having minor swallowing difficulties. There is evidence to support maintenance of swallowing function during and after treatment to prevent the deterioration of swallowing function (21,22). The speech pathology and dietetic departments in our institutions routinely see the patients during treatment to aid in swallowing therapy, gastrostomy education and monitoring nutritional status. Patients were encouraged to continue with oral intake and swallowing exercises during treatment despite having a gastrostomy in situ.
The retrospective nature of this study leads to a source of several potential biases. Firstly, there was a difference in population size between the PEG and RIG groups. There was heterogeneity with malignancies of differing stages and head and neck subsites in this study. Advanced staged tumours are associated with higher levels of dysphagia, and thus would be expected to have a longer duration of gastrostomy dependency. However, despite these potential biases, there was no significant difference in weight changes between patients with different stages. It was difficult to draw conclusions regarding weight changes and gastrostomy dependency between prophylactic and reactive gastrostomy patients as the reactive gastrostomy group did not include all head and neck cancer patients treated without gastrostomy tube insertions.
Gastrostomy use in head and neck cancer is an important and controversial topic among clinicians. The results from our study indicate optimisation of nutritional status prior to treatment is useful for patient’s weight maintenance and minimising weight loss post treatment. Protocol-based approach may be advantageous for patients with advanced malignancies due to greater potential for long-term dysphagia, however, patients with lower stage malignancy may not require gastrostomy tube insertion. The risks need to be stratified as these patients may benefit from a “watch and wait” approach with gradual escalation to short-term nasogastric feeds prior to gastrostomy tube insertion as nasogastric feeds have also been demonstrated to be a safe alternative for short-term dysphagia (15). Institution expertise, patient factors and preference must be taken into consideration prior to tube insertion.
Conclusions
- Gastrostomy insertions are important adjuncts in the maintenance of nutrition in head and neck cancer patients undergoing treatment.
- RIG tubes are associated with higher grade complications compared to percutaneous endoscopically inserted gastrostomy tubes.
- Prophylactic gastrostomy tube insertions were associated with shorter periods of gastrostomy dependency.
- Pre-treatment nutritional optimisation is important in the maintenance of higher post-treatment weight.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.09.03). EHO serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Human Research Ethics Committee at Flinders Medical Centre (HREC/16/SAC/261) and Royal Adelaide Hospital (HREC/16/RAH/211) granted ethics approval. Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v2, cancer incidence and mortality worldwide: IARC cancerbase No. 10. Lyon: International Agency for Research on Cancer Lyon, 2010.
- Nguyen NP, Sallah S, Karlsson U, et al. Combined chemotherapy and radiation therapy for head and neck malignancies: quality of life issues. Cancer 2002;94:1131-41. [Crossref] [PubMed]
- Orphanidou C, Biggs K, Johnston ME, et al. Prophylactic feeding tubes for patients with locally advanced head-and-neck cancer undergoing combined chemotherapy and radiotherapy—systematic review and recommendations for clinical practice. Curr Oncol 2011;18:e191-201. [Crossref] [PubMed]
- Kao SS, Peters MD, Krishnan SG, et al. Swallowing outcomes following primary surgical resection and primary free flap reconstruction for oral and oropharyngeal squamous cell carcinomas: A systematic review. Laryngoscope 2016;126:1572-80. [Crossref] [PubMed]
- Naidu MU, Ramana GV, Rani PU, et al. Chemotherapy-Induced and/or Radiation Therapy-Induced Oral Mucositis—Complicating the Treatment of Cancer. Neoplasia 2004;6:423-31. [Crossref] [PubMed]
- Bensadoun RJ, Riesenbeck D, Lockhart PB, et al. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer 2010;18:1033-8. [Crossref] [PubMed]
- Vissink A, Jansma J, Spijkervet FK, et al. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 2003;14:199-212. [Crossref] [PubMed]
- Grant DG, Bradley PT, Pothier DD, et al. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol 2009;34:103-12. [Crossref] [PubMed]
- International Union Against Cancer (UICC). TNM Classification of Malignant Tumours. 7th ed. Oxford: Wiley-Blackwell, 2009.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Kramer S, Newcomb M, Hessler J, et al. Prophylactic versus reactive PEG tube placement in head and neck cancer. Otolaryngol Head Neck Surg 2014;150:407-12. [Crossref] [PubMed]
- Chen AM, Li BQ, Lau DH, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2010;78:1026-32. [Crossref] [PubMed]
- Brown T, Banks M, Hughes BG, et al. Impact of early prophylactic feeding on long term tube dependency outcomes in patients with head and neck cancer. Oral Oncol 2017;72:17-25. [Crossref] [PubMed]
- Wang J, Liu M, Liu C, et al. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for patients with head and neck cancer: a systematic review. J Radiat Res 2014;55:559-67. [Crossref] [PubMed]
- Paleri V, Patterson J. Use of gastrostomy in head and neck cancer: a systematic review to identify areas for future research. Clin Otolaryngol 2010;35:177-89. [Crossref] [PubMed]
- Capuano G, Grosso A, Gentile PC, et al. Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant chemoradiotherapy. Head Neck 2008;30:503-8. [Crossref] [PubMed]
- Paccagnella A, Morello M, Da Mosto MC, et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer 2010;18:837-45. [Crossref] [PubMed]
- Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer 2004;91:447-52. [Crossref] [PubMed]
- Kendall KA, McKenzie SW, Leonard RJ, et al. Structural mobility in deglutition after single modality treatment of head and neck carcinomas with radiotherapy. Head Neck 1998;20:720-5. [Crossref] [PubMed]
- Tyml K, Mathieu-Costello O. Structural and functional changes in the microvasculature of disused skeletal muscle. Front Biosci 2001;6:D45-52. [Crossref] [PubMed]
- Kotz T, Federman AD, Kao J, et al. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Arch Otolaryngol Head Neck Surg 2012;138:376-82. [Crossref] [PubMed]
- Carnaby-Mann G, Crary MA, Schmalfuss I, et al. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012;83:210-9. [Crossref] [PubMed]
Cite this article as: Kao SST, Marshall-Webb M, Dharmawardana N, Foreman A, Ooi EH. Gastrostomy tube insertion outcomes in South Australian head and neck cancer patients. Aust J Otolaryngol 2018;1:21.