Turbo spin non echo-planar diffusion weighted MRI for cholesteatoma in revision mastoidectomy: a prospective study of diagnostic accuracy and clinical impact
Introduction
Treatment for cholesteatoma is surgery via mastoidectomy or an end-aural approach, except in the medically frail patient. A recognized major disadvantage of canal wall up mastoidectomy (as compared to a canal wall down procedure) was the high rate of recurrent or residual cholesteatoma, reported in 6–57% of cases, leading to the common practice of a ‘mandatory’ second-look procedure (1). In a more recent study, Neudert et al. report a similar residual and recurrence rates for canal wall up and canal wall down procedures (2). Therefore, although ‘mandatory’ second-look procedures have been common practice in patients who have undergone canal wall up surgery for cholesteatoma, it is now becoming increasingly difficult to justify this in asymptomatic patients (3). However, in cases where there is suspicion for residual or recurrent cholesteatoma, disease may not be easily detectable through standard clinical evaluations. Furthermore optimal pre-operative planning may be impossible if size and location of recurrent or persistent disease is not known.
With the aim of finding a radiological alternative to elective “second look” surgery, the diagnostic accuracy of multiple imaging techniques in the post-operative ear has been assessed in the published literature. Computed tomography (CT) of the temporal bones has a high negative predictive value (NPV) for identifying cholesteatoma only if the middle ear and mastoid are completely pneumatized and there is no abnormal soft tissue attenuation in these locations (4). In the post-operative ear, CT cannot distinguish well between residual cholesteatoma and other soft tissue pathology such as granulation, inflammation, fibrosis or effusions (5). Different MRI techniques have also been evaluated. Initially delayed gadolinium-enhanced T1-weighted MRI was found to have improved diagnostic accuracy as relative to CT, but this technique has been shown to be inaccurate for distinguishing cholesteatoma and fibrosis (4,6). Non-echo-planar diffusion weighted MRI (NEDWI) combined with T2 weighted imaging acquisition using HASTE (half-Fourier acquisition single-shot turbo spin-echo) has been shown to be more accurate in diagnosing cholesteatoma within the post-operative ear than the standard echo-planar diffusion weighted imaging (EPDWI) routinely used to identify acute stroke (7). The NEDWI technique permits spatial resolution of 2 mm and a lack of artefacts that make conventional EPDWI unhelpful in areas of the head containing air spaces and bone (8,9).
To date, diagnostic accuracy of the NEDWI technique has been variably reported as seen in Table 1 (10-20). A systematic review in 2011 evaluated NEDWI compared to EPDWI and in 8 studies including 207 patients prior to “second look” surgery, combined sensitivity for NEDWI was 91%, specificity 96%, positive predictive value 97% and NPV 85% (7). It was concluded in this review that although this was a promising diagnostic tool, further studies were needed (7). Studies performed since then have reported a range of sensitivities from 76–100% and specificities from 66.7–100% (10,15,17,19) (Table 1). However, in all but one of these studies, surgical findings, and not histopathology, were the gold standard. In addition, in some studies, the surgeon was either not blinded to the result of NEDWI or this detail was not reported (14,18,20).
Table 1
| Study | Year | Pro/Retro | n | Imaging | Scan-op time (months) | Reference standard | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV | NPV | Size limit (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Velthius et al. | 2014 | Retro | 21 | NEDWI | 3 | Surgeon findings | 76 | 100 | 100 | 50 | <5 |
| Garrido et al. | 2014 | Pro | 27 | NEDWI | N/A | Pathology | 100 [83–100] | 66.7 [22–95] | 91 [72–99] | 100 [38–100] | N/A |
| Profant et al. | 2012 | Pro | 17 | NEDWI (HASTE) | <6 | Surgeon findings | 96.15 (80.4–99.9) | 71.43 (29.04–96.3) | 92.59 (75.7–99.09) | 83.33 (35.9–99.58) | N/A |
| Illica et al. | 2012 | Pro | 5 | NEDWI (HASTE) | <1 | Surgeon findings | 94.1 | 100 | 100 | 0 | 4 |
| De Foer et al. | 2010 | Retro | 63 | NEDWI (HASTE) | <2 | Surgeon findings | 82.6 (74.8–88.3) | 87.2 (69.0–95.4) | 96 (89.7–98.5) | 56.5 (41.3–70.5) | N/A |
| Plouin-Gaudon et al. | 2010 | Pro | 17 | NEDWI (HASTE) | 11 | Surgeon findings | 62 | 88 | 89 | 58 | 4 |
| Rajan et al. | 2010 | Pro | 15 | NEDWI (HASTE) | <6 | Surgeon findings | 100 | 100 | 100 | 100 | 3 |
| Huins et al. | 2010 | Pro | 18 | NEDWI (HASTE) | 3 | Surgeon findings | 93.0 [75–99] | 100 [54–100] | 100 [86–100] | 75 [35–97] | 3 |
| Dhepnorrarat et al. | 2009 | Pro | 22 | NEDWI (HASTE) | <6 | Surgeon findings | 100 | 100 | 100 | 100 | 3 |
| De Foer et al. | 2008 | Pro | 19 | NEDWI (HASTE) | N/A | Surgeon findings | 90 | 100 | 100 | 96 | 2.5 |
| Dubrulle et al. | 2005 | Pro | 24 | NEDWI (HASTE) | <5 | Surgeon findings | 100 | 91 | 93 | 100 | 5 |
Pro/Retro, prospective or retrospective study design; Scan-op time, mean time between scan and operation in months; PPV, positive predictive value; NPV, negative predictive value; size limit, the largest size of residual cholesteatoma seen intraoperatively but reported as negative for cholesteatoma on MRI (a false negative). Velthuis et al. recorded a size limit of 5 mm given inaccurate description of size of cholesteatoma on pathology reports (10).
This introduces the possibility of bias in the reporting of diagnostic accuracy due to real or potential lack of independence of the reference and index tests as well as choice of an inappropriate gold standard in that surgically reported foci of cholesteatoma are likely to be less than those revealed at histopathological examination of material removed at surgery. In addition, there has been no study of the clinical impact on decision making of performance of NEDWI prior to surgery and in particular whether performance of NEDWI affects the decision to operate at all or the nature of the surgical procedure.
The aim of the current study was to:
- Prospectively determine the diagnostic accuracy of NEDWI in detecting residual or recurrent cholesteatoma with initial blinding of the operating surgeon to the imaging findings and;
- Assess the possible impact of NEDWI on the surgeon’s pre and intra-operative decision-making in cholesteatoma treatment.
Our hypothesis was that NEDWI is highly accurate for diagnosis of recurrent or residual cholesteatoma of the middle ear cleft including the epi- and hypotympanum. Furthermore, it was our secondary hypothesis that such a highly accurate imaging study would impact on surgical behaviour in two ways:
- By helping to avoid ‘unnecessary’ second look surgery in patients with no imaging evidence of cholesteatoma and no other clinical indication for surgery;
- By providing peri-operative planning information in cases where residual or recurrent disease is identified that would influence a surgeon’s intra-operative behaviour.
Methods
This study was a prospective, blinded, multicentre cohort study. Ethics approval was obtained from each institution’s human research ethics committee (HREC). The QUADAS tool was used to inform the study design (21). A sample size calculation was performed (22) using the expected sensitivity and specificity of 90% and a lower limit of the 95% confidence interval of 85%. To identify a clinically appropriate lower confidence limit, a survey of otolaryngology consultants was undertaken which asked the lowest sensitivity/specificity of an MRI the surgeon would accept for a clinically significant decision making tool. The survey responses ranged from 75–100% and the lower confidence limit of 85% was subsequently chosen to be effective. Assuming a normal approximation to the binomial distribution and using the above parameters, it was found that 100 patients would be required with an additional 10% (10 patients) to account for the possibility of loss of patients who underwent solely MRI or surgery following recruitment. Patients were recruited from the otolaryngology outpatient clinics of both Monash Health and Alfred Health networks in Melbourne, Australia. Included in the study were consecutive patients booked for second look mastoid surgery regardless of indication. Patients were excluded if they had a contraindication to MRI, were scheduled for MRI but did not have it for any reason, if they did not proceed to surgery, if informed consent could not be obtained, or if the surgical procedure was abandoned or cancelled.
Imaging protocol
A Siemens Avanto (Erlangen, Germany) 1.5T MRI scanner was used. Turbo spin non echo-planar diffusion weighted imaging (NEDWI) sequences were acquired in the coronal and axial plane using a 220 cm field of view and 2 values of the diffusion gradient: 0 and 1,000. The images were analysed and reported by a single, blinded consultant head and neck radiologist with experience and expertise in temporal bone imaging. The imaging report employed a standardized template. This included whether it was a positive or negative study, and, if positive for cholesteatoma, the size of the cholesteatoma and its anatomical location. Cholesteatoma is diagnosed when a mass with marked hyper-intensity (sometimes referred to as “light bulb brightness”) is seen in the middle ear cleft, external canal, attic, or mastoid cells when compared with the signal of brain tissue on NEDWI obtained with a b factor of 1,000 sec/mm2.
Surgical procedure
Each patient underwent their initial ‘second-look’ surgery according to the pre-operative clinical findings and plan. This would routinely involve a revision canal wall up tympanoplasty, further disease clearance or reconstructive procedure as indicated.
At the point at which the surgeon deemed the operation to be complete, but prior to closure of the wound, the pre-operative NEDWI MRI report was then revealed and the report was read to the still scrubbed surgeon. The surgeon was then allowed to perform further surgery as desired based on the findings in the report and his own clinical judgement, or proceed directly to wound closure.
Peri-operative surgeon surveys
In order to qualitatively assess the impact that knowledge of the results of a pre-operative NEDWI MRI might have on a surgeon’s decision making and behavior, a simple, pre-operative and post-operative survey was designed. The pre-operative survey (Figure 1) included questions with categorical and dichotomous responses, designed to elicit the clinical pre-test probability of the presence of cholesteatoma, and the influence of this probability on the surgeons’ decision to proceed to surgery. The post-operative survey (Figure 2) was again designed to elicit the clinical pre-test probability of the presence of cholesteatoma, but with the addition of the operative findings. It also examined any change in that probability once the MRI result was read by the surgeon (when the results of the MRI report were revealed after the surgery), and whether this additional knowledge had an impact on operative actions.
Patient flow
All patients in the study received NEDWI MRI imaging prior to surgery with the intention of surgery occurring within 6 weeks of imaging. This was thought to be an optimal time delay between imaging and surgery that would reduce the likelihood of interval development of cholesteatoma after imaging but before surgery that could confound our assessment of NEDWI diagnostic accuracy.
The patient and surgeon were blinded to the MRI result. The pre-operative survey was completed by the operating surgeon, immediately prior to the operation. The post-operative survey was completed by the surgeon immediately after the surgery was completed. A specimen was taken from the representative area of the operative cavity in every case and histology was reported. The pathologist was aware of the indication(s) for surgery but not the NEDWI MRI result. A positive diagnosis of cholesteatoma of any size on histology was the reference standard against which the imaging diagnosis (index test) was measured.
Data were maintained in a password protected database using Excel (Microsoft, USA). Stata IC 10.0 (College Station, TX, USA) was used for statistical analysis.
Results
Patients
The study was conducted at two institutions. Patients were recruited from one institution in the period between June 2011 and June 2015 and from the other between June 2012 and June 2015. Of an initial group of 31 recruited subjects, 6 were excluded (4 did not undergo surgery, 1 patient had no histology taken and 1 patient did not undergo MRI). In total there were 25 patients and 28 operations analysed, with 3 of the cases having repeat revisions, each revision being preceded by MRI. There were 7 females and 18 males aged 11–61 years (mean 38). There were 3 children and 22 adults. There was a median of 68 days from MRI to surgery (4–211 days). The median time between initial operation and second look operation was 641 days (366 to 3,492 days), or 1.76 years.
The survey response rates were 100% for the preoperative survey and 75% for the postoperative survey.
Non-EPI DWI MRI and histology both confirmed cholesteatoma in 5 cases (true positives) (Table 2). There were 2 false positive, 6 false negative and 15 true negative cases. Sensitivity was 45.45% (95% CI: 16.75–76.62%), specificity was 88.24% (95% CI: 63.56–98.54%), positive predictive value was 71.43% (95% CI: 29.04–96.33%) and NPV was 71.43% (95% CI: 47.82–88.72%). Overall accuracy was 71% (95% CI: 52.94–84.75%).
Table 2
| NEDWI findings | Cholesteatoma present (n) | Cholesteatoma absent (n) |
|---|---|---|
| NEDWI positive (n) | 5 | 2 |
| NEDWI negative(n) | 6 | 15 |
n, number of cases.
When subgroup analysis was performed on those with disease greater than 3 mm on histology, sensitivity was 80% (95% CI: 28.36–99.49%), specificity was 88.24% (95% CI: 63.56–98.54%), positive predictive value was 66.67% (95% CI: 22.28–95.67%) and NPV was 93.75% (95% CI: 69.77–99.84%).
The preoperative survey found that in 56% of cases, the surgeon’s pre-operative suspicion of cholesteatoma was ≤30% (i.e., very low suspicion, and in 28% of cases, suspicion of cholesteatoma was >85% (i.e., very high suspicion). The remaining 16% of cases were between 51–85% pretest probability clinically).
It also showed that the surgeon would have operated in 96% of cases even if shown a negative MRI for that patient (with an assumption that the MRI was >85% sensitive). In these cases, the indications for surgery were: persistent clinical concern for cholesteatoma, (36%), the need for ossicular chain reconstruction (48%), and tympanic membrane perforation or discharge (18%).
The postoperative survey found that after operative assessment and before MRI findings were revealed, the likelihood of cholesteatoma was reported as ≤30% in 74% of cases and >85% in 26% of cases. These results were unchanged when compared to those reported after the operative assessment and after the MRI findings were revealed to the surgeon intraoperatively following initial surgical exploration. The clinical course of the operation was changed by MRI in one patient, or 5.26% of cases, where the MRI findings prompted the surgeon to re-explore the pro-tympanum of the mastoid cavity. This disease had not been appreciated prior to reviewing the MRI report and images intra-operatively and this additional cholesteatoma was removed).
Although not part of our a priori study objectives, three of our study subjects had sub-clinical disease diagnosed in the contralateral ear. In one case, the patient became symptomatic in that ear later and was treated accordingly. The other cases were asymptomatic and subsequently managed with serial MRIs to monitor disease progression.
Discussion
The diagnostic accuracy of non-EPI DWI MRI held true to the previously published literature only when cholesteatoma size greater than 3 mm were analysed. When all cases including five cases under 3 mm were analysed, the diagnostic accuracy was significantly lower than other reported data.
In our cohort of patients, Non-EPI DWI MRI identified 5 true positives and 2 false positives. The 2 total false positives were both reported as being <5 mm in size, and were likely due to partial volume averaging of the signal compared to nearby darker tissue. Of the 6 total false negatives, 5 were disease under 3 mm. In 4 of these 5 the surgeon had suspected the presence of disease. The other false negative was a cholesteatoma comprising an epithelial lining without matrix contents. It may be that the lack of material within the epithelial lining reduced the diffusion restriction produced by the lesion and thus its visibility.
Thus, using a 1.5T scanner and NEDWI sequence, the caveat that disease less than 3mm may be missed is evident. This is consistent with current literature as seen in Table 1. While improved signal to noise and spatial resolution may be achieved with a 3 Tesla scanner, a recent study of 39 patients with suspected cholesteatoma by Lincot et al. found no difference in diagnostic accuracy of NEDWI when performed at 3Tesla when compared with 1.5T strength NEDWI (23).
The question remains whether the level of diagnostic accuracy of NEDWI as measured in our study, and that which exists in the literature overall, is sufficient to change the decision-making of clinicians, which may be influenced by other issues such as lack of belief in the safety of non-operative surveillance strategies and financial incentives that favour surgery.
In our study, there was little impact of an accurate diagnostic test on clinical decision-making regarding post canal wall up mastoidectomy patients, with the overwhelming majority (93%), requiring second look surgery in the opinion of the operating surgeon, despite a negative MRI result.
In approximately one third of patients, who had no other indication for surgery but surveillance, we would have expected that a negative NEDWI may have altered this decision and the reason(s) for the lack of influence on decision making in this situation are unclear as these were not specifically elicited by our survey instrument.
In the other two thirds of patients, the NEDWI result was largely irrelevant as the indication for surgery was for non-cholesteatoma reasons such as ossicular chain reconstruction, or myringoplasty.
Thus, the Non-EPI DWI MRI has its greatest potential clinical impact and cost savings, in terms of reducing unnecessary surgery when negative, and when there is no other clinical indication for revision ear surgery. We suggest a follow up non-EPI DWI MRI at one year given the limited NPV noted in this study.
We found that NEDWI may be useful to direct the surgeon to find a small cholesteatoma that would otherwise be missed; however, this occurred in only one patient, making it questionable whether this justifies the cost of performing NEDWI as a routine in all patients scheduled for second look surgery. In addition, three other patients had asymptomatic disease identified in the contralateral ear. In one case, this became symptomatic and was treated. It was beyond the scope of our study to determine whether surveillance of the unoperated ear, which does not add to the cost or duration of the MRI, improved overall patient management.
In additional to the post-operative Canal-wall-up mastoidectomy patients, NEDWI has potential applications in other clinical groups such as post-mastoid obliteration patients (24).
A limitation of this study is the inherent bias introduced with the pre-operative and post-operative surveys. We attempted to reduce the impact of this bias by ensuring the surgeon completed the pre-operative survey well before the commencement of the operation, and the post-operative survey was completed immediately after the operation. The NEDWI MRI report was revealed after the surgeon verbalized that the operation was complete, so that surgical decision making could not be influenced by the report. In addition, a single surgeon performing all cases allowed a level of consistency in decision making logic.
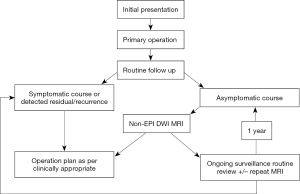
We propose the use of non-EPI DWI as the imaging modality of choice to firstly diagnose and localize residual or recurrent disease and to distinguish it from non-cholesteatoma changes that can appear identical on both CT and standard MRI that does not employ NEDWI techniques. It is our view that both symptomatic and asymptomatic populations should undergo MRI and current literature suggests 6–12 monthly surveillance NEDWI is appropriate, given the natural history of the disease process and lesser sensitivity of NEDWI for cholesteatoma deposits less than 3 mm in size (15). We consider yearly MRI and clinical surveillance alone as the optimal management pathway for postoperative, asymptomatic canal wall up patients with no other indication for second look surgery (Figure 3). We propose two post-operative MRI scans, each a year apart. The second scan is to identify any small recurrences that might have been missed on the first MRI one year post surgery, given the diagnostic size limitations identified in the current paper. This management plan is complementary to the clinical pathways proposed by Keeler et al. in a recently published literature review (3), and could reduce the number of unnecessary ‘mandatory’ second-look procedures.
Strengths of the study include its prospective design, independence of the index test (MRI) and reference standards (surgery, histology), initial blinding of the operating surgeon to the MRI result, the use of histology rather than surgery alone as a reference standard in all cases, and inclusion of subjects on the basis of their need for surgery and not as a result of an MRI finding. Limitations of the study included a relatively long time lapse in some cases between MRI and surgery that could have resulted in growth of cholesteatoma during that interval, reducing the sensitivity of MRI, and the use of a single surgeon for the clinical impact surveys. Although the study period spanned 4 years, recruitment of eligible patients was challenging, resulting in a failure to achieve the desired sample size, resulting in widened 95% confidence intervals around our point estimate of sensitivity and specificity of NEDWI for cholesteatoma. Furthermore, the nature of the study design, in order to ensure histology in every case, may have resulted in selection bias and an unrepresentative patient population. Finally, the tertiary hospital otology services where this study was conducted are more likely to have patients with indications for second look surgery other than simple surveillance of disease, such as ossicular chain reconstruction, than would otorhinolaryngology non-tertiary practice and this may have resulted in biased estimation of the influence of NEDWI on surgical decision-making.
Avenues for further research in this patient group include surveillance of asymptomatic patients with serial MRI to assess the clinical course of an asymptomatic cholesteatoma recurrence in an operated ear and also the natural history of progression of cholesteatoma where it is found in the contralateral ear where no operation has been performed. This would help to inform the optimum interval between surveillance NEDWI studies, potentially saving costs. Further clinically relevant research topics relating to appropriate utilization of NEDWI include the appropriate duration of clinical follow up in an asymptomatic post-operative patient and an assessment of an appropriate surveillance/management pathway in the paediatric population.
Conclusions
Our study demonstrated a similar diagnostic accuracy for NEDWI in the diagnosis of cholesteatoma in the post canal-wall up ear, to that reported previously in the literature in other cohorts of patients, with sensitivity falling as size decreases below 3 mm. However, performance of NEDWI had a low impact on the clinical decision-making process in our cohort of patients, in part due to the high proportion of patients who were being offered surgery due to the need for myringoplasty and ossicular chain reconstruction rather than surveillance of disease alone. We believe NEDWI is sufficiently accurate to be used as a “radiological second look” in the asymptomatic postoperative ear, the asymptomatic but diseased contralateral ear and in an obliterated mastoid ear.
Acknowledgments
Paper presented at: Australian Society of Otolaryngology Head and Neck Surgery (ASOHNS) Annual Scientific Meeting Melbourne 2016; Royal Australasian College of Surgeons Annual Scientific Meeting Victoria and Tasmania, Hobart 2015 - RC Bennett Award for the best clinical research paper.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.02.02). PP serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation (Southern health and Alfred Health Ethics Committee, Melbourne, Australia) and with the Helsinki Declaration (as revised in 2013). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parisier SC, Hanson MB, Han JC, et al. Pediatric cholesteatoma: an individualized, single-stage approach. Otolaryngol Head Neck Surg 1996;115:107-14. [Crossref] [PubMed]
- Neudert M, Lailach S, Lasurashvili N, et al. Cholesteatoma recidivism: comparison of three different surgical techniques. Otol Neurotol 2014;35:1801-8. [Crossref] [PubMed]
- Keeler JA, Kaylie DM. Cholesteatoma: Is a second stage necessary? Laryngoscope 2016;126:1499-500. [Crossref] [PubMed]
- De Foer B, Nicolay S, Vercruysse JP, et al. Imaging of Cholesteatoma. Temporal Bone Imaging: Springer, 2014:69-87.
- Blaney SP, Tierney P, Oyarazabal M, et al. CT scanning in "second look" combined approach tympanoplasty. Rev Laryngol Otol Rhinol (Bord) 2000;121:79-81. [PubMed]
- Vanden Abeele D, Coen E, Parizel PM, et al. Can MRI replace a second look operation in cholesteatoma surgery? Acta Otolaryngol 1999;119:555-61. [Crossref] [PubMed]
- Jindal M, Riskalla A, Jiang D, et al. A systematic review of diffusion-weighted magnetic resonance imaging in the assessment of postoperative cholesteatoma. Otol Neurotol 2011;32:1243-9. [Crossref] [PubMed]
- De Foer B, Vercruysse JP, Bernaerts A, et al. The value of single-shot turbo spin-echo diffusion-weighted MR imaging in the detection of middle ear cholesteatoma. Neuroradiology 2007;49:841-8. [Crossref] [PubMed]
- De Foer B, Vercruysse JP, Pilet B, et al. Single-shot, turbo spin-echo, diffusion-weighted imaging versus spin-echo-planar, diffusion-weighted imaging in the detection of acquired middle ear cholesteatoma. AJNR Am J Neuroradiol 2006;27:1480-2. [PubMed]
- Velthuis S, van Everdingen KJ, Quak JJ, et al. The value of non echo planar, diffusion-weighted magnetic resonance imaging for the detection of residual or recurrent middle-ear cholesteatoma. J Laryngol Otol 2014;128:599-603. [Crossref] [PubMed]
- De Foer B, Vercruysse JP, Bernaerts A, et al. Detection of postoperative residual cholesteatoma with non-echo-planar diffusion-weighted magnetic resonance imaging. Otol Neurotol 2008;29:513-7. [Crossref] [PubMed]
- De Foer B, Vercruysse JP, Spaepen M, et al. Diffusion-weighted magnetic resonance imaging of the temporal bone. Neuroradiology 2010;52:785-807. [Crossref] [PubMed]
- Dhepnorrarat RC, Wood B, Rajan GP. Postoperative non-echo-planar diffusion-weighted magnetic resonance imaging changes after cholesteatoma surgery: implications for cholesteatoma screening. Otol Neurotol 2009;30:54-8. [Crossref] [PubMed]
- Dubrulle F, Souillard R, Chechin D, et al. Diffusion-weighted MR imaging sequence in the detection of postoperative recurrent cholesteatoma. Radiology 2006;238:604-10. [Crossref] [PubMed]
- Garrido L, Cenjor C, Montoya J, et al. Diagnostic capacity of non-echo planar diffusion-weighted MRI in the detection of primary and recurrent cholesteatoma. Acta Otorrinolaringol Esp 2015;66:199-204. [Crossref] [PubMed]
- Huins CT, Singh A, Lingam RK, et al. Detecting cholesteatoma with non-echo planar (HASTE) diffusion-weighted magnetic resonance imaging. Otolaryngol Head Neck Surg 2010;143:141-6. [Crossref] [PubMed]
- Ilıca AT, Hıdır Y, Bulakbaşı N, et al. HASTE diffusion-weighted MRI for the reliable detection of cholesteatoma. Diagn Interv Radiol 2012;18:153-8. [PubMed]
- Plouin-Gaudon I, Bossard D, Ayari-Khalfallah S, et al. Fusion of MRIs and CT scans for surgical treatment of cholesteatoma of the middle ear in children. Arch Otolaryngol Head Neck Surg 2010;136:878-83. [Crossref] [PubMed]
- Profant M, Slavikova K, Kabatova Z, et al. Predictive validity of MRI in detecting and following cholesteatoma. Eur Arch Otorhinolaryngol 2012;269:757-65. [Crossref] [PubMed]
- Rajan GP, Ambett R, Wun L, et al. Preliminary outcomes of cholesteatoma screening in children using non-echo-planar diffusion-weighted magnetic resonance imaging. Int J Pediatr Otorhinolaryngol 2010;74:297-301. [Crossref] [PubMed]
- Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [Crossref] [PubMed]
- Flahault A, Cadilhac M, Thomas G. Sample size calculation should be performed for design accuracy in diagnostic test studies. J Clin Epidemiol 2005;58:859-62. [Crossref] [PubMed]
- Lincot J, Veillon F, Riehm S, et al. Middle ear cholesteatoma: Compared diagnostic performances of two incremental MRI protocols including non-echo planar diffusion-weighted imaging acquired on 3T and 1.5T scanners. J Neuroradiol 2015;42:193-201. [Crossref] [PubMed]
- Vercruysse JP, De Foer B, Pouillon M, et al. The value of diffusion-weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: a surgical verified study of 100 patients. Eur Radiol 2006;16:1461-7. [Crossref] [PubMed]
Cite this article as: Paddle P, Hurtado G, Goergen S, Hong S, Baxter M, Copson B. Turbo spin non echo-planar diffusion weighted MRI for cholesteatoma in revision mastoidectomy: a prospective study of diagnostic accuracy and clinical impact. Aust J Otolaryngol 2018;1:12.