
Metastatic basal cell carcinoma: a review of six cases
Introduction
Basal cell carcinomas (BCCs) are locally invasive skin neoplasms of cells in the epidermal basal layer, representing 70% of all skin cancers in Australia, with a 30% lifetime risk of development (1). Whilst BCC metastasis has a documented incidence of only 0.0028% to 0.5% (2,3), there is significant morbidity and mortality associated with such an event. The most likely locations of metastasis are to the lungs, bone and lymph nodes, via both lymphatic and hematogenous spread (2,4). The majority of metastasizing BCCs originate in the head and neck, with evidence that over 65% of BCCs arise from the face alone (5).
Although metastatic BCCs are a rare occurrence, there has been evidence to suggest that there are certain features that may be used to predict metastatic spread. These include histological subtype, size, and perineural or lymphovascular invasion. Here, we aim to analyse features of primary and metastatic BCCs, and identify possible predictors of spread in the primary lesions.
Methods
We reviewed six cases of metastatic BCC, which were seen in our institution (Royal North Shore Hospital, Sydney, Australia) between the years 2000 and 2016. The pathology reports of the original tumours were obtained and, where possible, the pathological slides from the primary tumour were reviewed. The pathological slides of all metastases were also independently reviewed by a second subspecialist surgical pathologist and, if required, additional immunohistochemistry (p53, SMA, BCL2, Ki67) was performed to confirm the diagnosis of metastatic BCC. The patients’ histories, including age, gender and relevant comorbidities, especially immunosuppressive risk factors (history of cancer, poorly controlled diabetes and immunosuppressive medications) were also reviewed, as was the time between identification of primary BCC and detection of metastasis.
Cases
Case 1
A 66-year-old female first noticed a BCC to the left side of the nose in 1990. This was managed with cryotherapy at the time. A biopsy taken one year later showed ongoing disease, but she delayed further treatment due to social reasons and was then lost to follow up. In 2007, she presented with a very large, deeply infiltrative rodent ulcer of the left half of the face, obliterating the anterior eye, medial and lateral canthus, supraorbital skin, nasolabial fold and entire medial aspect of the nose. This lesion was found to virtually obliterate the globe, tracking inferiorly through the posterior nasal structures and maxilla to the hard palate. Histological examination revealed an infiltrative BCC with focal areas of necrosis, focal squamous differentiation, and perineural involvement. This was managed with surgical excision and radiotherapy to the nose and face. Three years later, a PET scan & MRI demonstrated two nodules in the lower lobe of the right lung. Core biopsy confirmed metastatic BCC with similar morphology to that seen in the primary. This was managed with pulmonary radiotherapy and targeted therapy, utilizing a novel hedgehog pathway inhibitor, to which a partial response was obtained.
Case 2
A 68-year-old male with a history of multiple solar elastoses initially presented with an invasive BCC of the back, of nodular/morpheaform subtype, infiltrating into the lower third of the reticular dermis. It demonstrated small nests of epithelial cells within focally sclerotic stroma. There was no angiolymphatic or perineural invasion identified. This was removed by surgical excision. Two years later, a CT lumbar spine revealed ill-defined, mixed lytic and sclerotic densities in the posterior aspect of the right ilium, first sacral segment and bilateral sacral ala. A core biopsy confirmed metastatic BCC demonstrating similar morphology to that seen in the primary tumour. Oval to spindle shaped nuclei were found with some palisading around nests & acinar spaces within nests. It showed a partial response to radiotherapy and targeted therapy with a hedgehog pathway inhibitor.
Case 3
A 79-year-old male presented with a lesion of the right forehead, exhibiting an irregular area of nodular thickening in the centre. Histological analysis revealed it to be a morphoeic BCC, with infiltration through the dermis and into the subcutis. There was no lymphovascular invasion or perineural spread. Over the following decade, he returned with multiple localized BCCs, including an infiltrative BCC of the right parotid region, a nodular & infiltrative BCC of the left back and an infiltrative BCC of the right cheek. Subsequent to this, he re-presented with a suspicious node in the right submandibular region. A core biopsy confirmed BCC with morphology similar to that seen previously, but none associated in soft tissue. Although it was difficult to classify and immunohistochemistry for BerEP4 and BCL2 was ambiguous, on review it was thought more likely to represent metastatic BCC than a new primary cutaneous BCC.
Case 4
A 56-year-old man presented with an ulcerated lesion of the right ear. Histology demonstrated a nodular BCC that infiltrated into the margin of the meatus, not involving cartilage. However, the excision margins appeared incomplete, and a revision was performed later that year. The re-excised lesion demonstrated similar pathology, but appeared incompletely excised once again. The patient was unfortunately lost to follow-up, until 3 years later when he returned with a recurrence of the BCC. He was assessed and scheduled for temporal bone resection, but did not follow-up as he was not keen on surgery. Eight years later, the patient presented with a large fungating mass of the right ear. MRI showed marked involvement of the right ear and petrous temporal bone, including the mastoid and zygoma. The patient underwent right ear and temporal bone excision and petrous apicectomy, this time with clear margins. Histological analysis showed an extensive BCC with ulceration, of morpheaform & infiltrative morphology. It was 30 mm deep, extending to involve the deep fascia, cartilage and parotid. It had widespread necrosis, and demonstrated perineural invasion of the facial nerve, but no blood vessel invasion. There was no squamous dysplasia. A PET scan at the time had demonstrated not only the large FDG-avid lesion of the right ear and temporal bone, but also increased uptake at multiple spinal levels and the 8th right rib. Core biopsy of the bone confirmed metastatic BCC with extensive necrosis. The patient underwent radiotherapy to the parotid and base of skull & neck following surgical excision, with palliative radiotherapy to the spine.
Case 5
A 52-year-old male flight attendant presented for review with a lesion to the left anterior chest wall with a centrally ulcerated nodule. Initially, this was thought to be a tropical ulcer secondary to an insect bite sustained on a visit to India one month prior. However, it exhibited rapid growth with purulent ooze. This was found to be an infiltrating BCC invading into the subcutis. There was no perineural or lymphovascular invasion seen. Two years later, he was found to have multiple pulmonary nodules, confirmed on PET scan. Core biopsy demonstrated metastatic BCC with similar morphology to that seen in the primary tumour. Photomicrographs of the lung biopsy are shown in Figure 1. The patient was subsequently lost to follow-up.
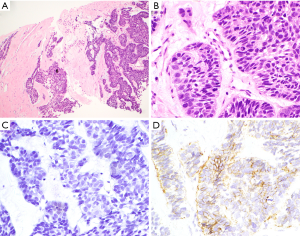
Case 6
A 42-year-old male presented with a BCC of the left axilla with some minor metatypical (squamoid) differentiation. This was managed by excision of the axillary lesion and lymph node clearance (0/19 nodes involved). In the subsequent years, he developed further BCCs that were managed surgically, including a multifocal superficial BCC of the right outer canthus, an upper back invasive papulonodular BCC and a recurrence of the original axillary BCC. Although his clinical course was uncomplicated, he re-presented eight years later with multiple lung nodules and a parasternal mass on CT chest. Biopsies of these masses confirmed metastatic BCC with similar morphology to the upper back cutaneous lesion. Further management was suspended, as the patient was lost to follow-up.
A comparison of the clinical and histological features of the cases is presented in Table 1.
Table 1
| Case | Age | Site of primary | Histological subtype of primary | Suspected duration of lesion (years) | Site of metastasis | Presence of squamous differentiation in metastasis | Perineural and/or lymphovascular invasion in primary | Size of primary (length × width × depth, mm) | Number of excisions required | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | Face | Infiltrative, with squamous differentiation | 17 | Lung | Present | Perineural | 55×40×40 | 1 | 0 |
| 2 | 68 | Back | Nodular/morpheaform | 2 | Sacrum & ilium | Absent | Absent | 20×15×2.1 | 1 | 0 |
| 3 | 79 | Forehead | Morphoeic BCC | 7 | Submandibular | Absent | Absent | 10×11×7.5 | 1 | 0 |
| 4 | 56 | Ear | Morpheaform & infiltrative | 11 | Spine & ribs | Absent | Perineural | 45×35×30 | 3 | 2 |
| 5 | 52 | Chest wall | Infiltrating | 2 | Lung | Absent | Absent | 85×40×13 | 1 | 0 |
| 6 | 42 | Back | Papulonodular | 8 | Lung | Present | Absent | Unavailable | 1 | 0 |
BCC, basal cell carcinoma.
Discussion
Although BCC represents the most common cutaneous malignancy, metastatic BCC remains a rare phenomenon. It has been postulated that certain clinical or histopathological features of BCCs may serve as indicators for the propensity to metastasize. Traditionally, BCCs have been divided into two categories: indolent and aggressive; the indolent variants comprising nodular and superficial BCCs, and the aggressive ones comprising morpheaform, infiltrating and micronodular subtypes (6). The most common clinical variant of BCCs is the nodular BCC, with histopathology characterised by discrete nests of basaloid cells in the dermis, with peritumoral retraction from a stroma that exhibits myxoid change, and peripheral palisading of cells. Single cell necrosis, plump fibroblasts and a high mitotic rate are uncommon (6,7). In contrast, the more aggressive morpheaform variant consists of thick columns of basaloid cells that extend into a densely collagenous stroma containing plump fibroblasts. There is more individual cell necrosis and mitotic activity, and a higher propensity for invasion into the reticular dermis (7). With increasing aggressiveness, as seen in the infiltrative subtype, there is marked stromal fibrosis with dense collagen bundles, and abundant proplastic fibroblasts. These more aggressive subtypes present an increased risk of invasion into adjacent skin layers, as well as perineural invasion. Despite this traditional classification, up to one third may exhibit an admixture of variants.
The most common primary histological subtypes in the six cases reviewed were morpheaform & infiltrative, with mixed histology frequently present. Evidently, aggressive histological subtypes were predominant in the BCC primaries. This was accompanied by perineural and/or lymphovascular invasion in two of the cases. This lends support to the traditional thinking that BCCs of the more aggressive subtypes are more likely to metastasize (4), and possibly also those with perineural spread and blood vessel invasion (2,4,8).
It has also been suggested that larger BCCs, and BCCs of a higher TNM stage (> T2) are also more likely to metastasize (5). A review of 238 cases by Snow et al. suggested that tumours larger than 30 mm in diameter had a 2% incidence of metastasis, compared to the overall rate of <1% for morpheaform BCCs. Furthermore, the data demonstrated that 80% of metastatic BCCs arose from tumours larger than 50 mm in diameter. Our cases reviewed here showed metastatic BCCs arising from large lesions (greater than approximately 50 mm in diameter) in 3 of the cases (with unavailable records in one case); the largest lesion being 85 mm × 40 mm. The other 2 cases demonstrated smaller primaries from around 15–25 mm in diameter. Of interest was that the size of the lesion did not necessarily predict recurrence at the same site, with the majority being cleared by a single excision with or without adjuvant radiotherapy.
A certain type of BCC exhibits a combination of both BCC and SCC features – known as basosquamous carcinoma or metatypical BCC. It is histologically characterized by infiltrating jagged strands of tumour cells, with relatively uniform nuclei and peripheral palisading (although this is not always seen) (8,9). Within these nests, some cells exhibit a loss of basaloid differentiation and a progression to squamous differentiation, characterized by a more eosinophilic cytoplasm and keratinization. Basosquamous carcinomas exhibit more atypical mitoses, cell pleomorphism and nuclear hyperchromatism (8), and are clinically more locally invasive. The question of what pathological features should define a basosquamous carcinoma, and whether basosquamous carcinomas truly are a variant of BCCs, or whether they should be classified as a separate lesion with different behaviour has been a topic of controversy in the past (10,11). It has been presumed that BCCs with elements of squamous differentiation are more likely to metastasize, but some studies have observed that there is often very little squamous differentiation in the histology of any of the primaries (2), suggesting that metastatic potential may not be solely predicted by squamous differentiation. Of our six cases reviewed, one was found to have squamous differentiation in both the primaries and metastases. One other case was noted to have an axillary BCC primary with squamous differentiation, but was the unlikely primary lesion giving rise to a lung metastasis, as the morphology of the lung lesion more resembled that of a prior papulonodular BCC primary of the back. Several studies have examined the behaviour of basosquamous carcinomas, and found them to be more aggressive with a higher rate of recurrence when compared with BCCs lacking metatypical features (10,12-14). As such, although the majority of metastatic BCCs do not appear to arise from basosquamous types, the diagnosis of a BCC with squamous differentiation on original biopsy carries both prognostic and management implications. Complete surgical excision with negative margins, with consideration of radiotherapy if this cannot be achieved, and ongoing follow-up for recurrence are essential to the management strategy of basosquamous carcinomas. Although there has been no consensus on the appropriate excision margins for basosquamous carcinomas, one study suggested a 3–5 mm margin for head and neck lesions, and 5–10 mm margins at other areas (although a 10% rate of recurrence was still found with these margins) (12).
Other clinical features which have been thought to be risk factors for metastases include: male gender (with a male:female ratio of 2:1) (2), and location in the head and neck region (2). Previous authors have suggested that the frequency of metastasis from the head and neck region may be associated with the thin skin of the scalp and high concentration of large-calibre vessels (15). Five out of the six cases reviewed involved male patients, and three of the six involved BCCs originating from head and neck primaries, including the face, forehead and ear (although our institution as a tertiary head and neck referral centre may have been a source of bias). Of particular interest was the propensity for metastasis to the lungs, a well-documented phenomenon of metastatic BCCs which drastically reduces survival in comparison to metastases limited to lymph nodes (16-19). It was also noted that local relapse was observed in only one of the cases that received treatment.
There have been several immunohistochemical markers which have been identified as possible indicators of BCC metastatic potential. Of these, the tumour suppressor p53 has arguably been the most widely researched given the evidence that chronic sun exposure is an important risk factor to p53 mutation and carcinogenesis (20). Other markers that have been examined include D2–40 (a monoclonal antibody classic to fibrous histiocytomas), Ki-67 (a marker of cell proliferation) (21,22), the anti-apoptotic gene Bcl-2 (18,23,24), and smooth muscle actin (SMA).
Most studies regarding p53 in BCCs have found a significant upregulation of p53 in aggressive variants, compared with non-aggressive (25-28). However, whether this correlates to increased metastatic potential is not clear (18). Further studies have examined the role of Ki-67 with equivocal results: whilst studies concur on the role of Ki-67 in proliferative activity, the correlation between aggressive histological subtypes and Ki-67 expression is less clear (22,29). Furthermore, there is no defined association between Ki-67 and tumour size, which has been shown to be a predictor of metastatic potential (5,22). Although the majority of BCCs are positive for Bcl-2, there is no clear link between Bcl-2 and tumour aggressiveness or potential for invasion (18,23,24,28). Perhaps the newest immunohistochemical marker with greatest potential to predict metastasis is alpha-SMA: typically used as a marker of myofibroblasts, and in the detection of pancreatic cancer. A number of studies have found that aggressive BCCs tend to express alpha SMA in the stroma, whilst non-aggressive BCCs express it in tumour cells themselves (30-32).
At the time of writing it appears that the primary role of immunohistochemistry is to confirm the diagnosis of BCC, particularly in the setting of metastasis, and exclude other more aggressive lesions in the differential diagnosis, including melanoma and Merkel cell carcinoma; and there are no validated markers which predict an increased risk of metastasis.
Conclusions
Our review of six cases of metastatic BCCs confirms that metastasis appears most likely in large BCCs, BCCs with vascular space invasion or perineural spread and tumours with some metatypical squamoid differentiation. This has implications for treatment and prognosis, requiring careful caution at first presentation and diligent excision to ensure negative margins. In support of previous data, the primaries of metastatic BCCs preferentially arise from the head and neck region, which should further promote careful surveillance from initial presentation. Whilst males tend to predominate, this may just reflect the higher incidence of primary BCCs in males.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.09.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lear JT, Smith AG. Basal cell carcinoma. Postgrad Med J 1997;73:538-42. [Crossref] [PubMed]
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literature. J Am Acad Dermatol 1984;10:1043-60. [Crossref] [PubMed]
- Malone JP, Fedok FG, Belchis DA, et al. Basal cell carcinoma metastatic to the parotid: report of a new case and review of the literature. Ear Nose Throat J 2000;79:511-5, 518-9. [PubMed]
- Ting PT, Kasper R, Arlette JP. Metastatic basal cell carcinoma: report of two cases and literature review. J Cutan Med Surg 2005;9:10-5. [Crossref] [PubMed]
- Snow SN, Sahl W, Lo JS, et al. Metastatic basal cell carcinoma. Report of five cases. Cancer 1994;73:328-35. [Crossref] [PubMed]
- Crowson AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod Pathol 2006;19:S127-47. [Crossref] [PubMed]
- Marzuka AG, Book SE. Basal Cell Carcinoma: Pathogenesis, Epidemiology, Clinical Features, Diagnosis, Histopathology, and Management. Yale J Biol Med 2015;88:167-79. [PubMed]
- Lima NL, Verli FD, de Miranda JL, et al. Basosquamous carcinoma: histopathological features. Indian J Dermatol 2012;57:382-3. [Crossref] [PubMed]
- Johnson BF, Moore PJ, Goepel JR, et al. Basosquamous carcinoma, a wolf in sheep's clothing? Report of 3 cases. Postgrad Med J 1989;65:750-1. [Crossref] [PubMed]
- Garcia C, Poletti E, Crowson AN. Basosquamous carcinoma. J Am Acad Dermatol 2009;60:137-43. [Crossref] [PubMed]
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (Basosquamous Carcinoma) of head and neck. Otolaryngol Pol 2012;66:419-23. [Crossref] [PubMed]
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res 2008;27:65. [Crossref] [PubMed]
- Martin RC 2nd, Edwards MJ, Cawte TG, et al. Basosquamous carcinoma: analysis of prognostic factors influencing recurrence. Cancer 2000;88:1365-9. [Crossref] [PubMed]
- Cohen PR, Schulze KE, Nelson BR. Basal cell carcinoma with mixed histology: a possible pathogenesis for recurrent skin cancer. Dermatol Surg 2006;32:542-51. [PubMed]
- Dzubow LM. Metastatic basal cell carcinoma originating in the supra-parotid region. J Dermatol Surg Oncol 1986;12:1306-8. [Crossref] [PubMed]
- Robinson JK, Dahiya M. Basal cell carcinoma with pulmonary and lymph node metastasis causing death. Arch Dermatol 2003;139:643-8. [Crossref] [PubMed]
- Seo SH, Shim WH, Shin DH, et al. Pulmonary metastasis of Basal cell carcinoma. Ann Dermatol 2011;23:213-6. [Crossref] [PubMed]
- Ionescu DN, Arida M, Jukic DM. Metastatic basal cell carcinoma: four case reports, review of literature, and immunohistochemical evaluation. Arch Pathol Lab Med 2006;130:45-51. [PubMed]
- Icli F, Uluoglu O, Yalav E, et al. Basal cell carcinoma with lung metastases: a case report. J Surg Oncol 1986;33:57-60. [Crossref] [PubMed]
- Ouhtit A, Nakazawa H, Armstrong BK, et al. UV-radiation-specific p53 mutation frequency in normal skin as a predictor of risk of basal cell carcinoma. J Natl Cancer Inst 1998;90:523-31. [Crossref] [PubMed]
- Baum HP, Meurer I, Unteregger G. Ki-67 antigen expression and growth pattern of basal cell carcinomas. Arch Dermatol Res 1993;285:291-5. [Crossref] [PubMed]
- Bieniek A, Pula B, Piotrowska A, et al. Expression of metallothionein I/II and Ki-67 antigen in various histological types of basal cell carcinoma. Folia Histochem Cytobiol 2012;50:352-7. [Crossref] [PubMed]
- Ramdial PK, Madaree A, Reddy R, et al. bcl-2 protein expression in aggressive and non-aggressive basal cell carcinomas. J Cutan Pathol 2000;27:283-91. [Crossref] [PubMed]
- Giri DD, Gupta PK, Hoda RS. Cytologic diagnosis of metastatic basal cell carcinoma. Report of a case with immunocytochemical and molecular pathologic considerations. Acta Cytol 2000;44:232-6. [Crossref] [PubMed]
- Auepemkiate S, Boonyaphiphat P, Thongsuksai P. p53 expression related to the aggressive infiltrative histopathological feature of basal cell carcinoma. Histopathology 2002;40:568-73. [Crossref] [PubMed]
- Ansarin H, Daliri M, Soltani-Arabshahi R. Expression of p53 in aggressive and non-aggressive histologic variants of basal cell carcinoma. Eur J Dermatol 2006;16:543-7. [PubMed]
- Barrett TL, Smith KJ, Hodge JJ, et al. Immunohistochemical nuclear staining for p53, PCNA, and Ki-67 in different histologic variants of basal cell carcinoma. J Am Acad Dermatol 1997;37:430-7. [Crossref] [PubMed]
- Mateoiu C, Pirici A, Bogdan F. Immunohistochemical nuclear staining for p53, PCNA, Ki-67 and bcl-2 in different histologic variants of basal cell carcinoma. Rom J Morphol Embryol 2011;52:315-9. [PubMed]
- Kramer E, Herman O, Frand J, et al. Ki67 as a biologic marker of basal cell carcinoma: a retrospective study. Isr Med Assoc J 2014;16:229-32. [PubMed]
- Adegboyega PA, Rodriguez S, McLarty J. Stromal expression of actin is a marker of aggressiveness in basal cell carcinoma. Hum Pathol 2010;41:1128-37. [Crossref] [PubMed]
- Bozdogan O, Erkek E, Atasoy P, et al. Bcl-2-related proteins, alpha-smooth muscle actin and amyloid deposits in aggressive and non-aggressive basal cell carcinomas. Acta Derm Venereol 2002;82:423-7. [Crossref] [PubMed]
- Pilloni L, Bianco P, Manieli C, et al. Immunoreactivity for alpha-smooth muscle actin characterizes a potentially aggressive subgroup of little basal cell carcinomas. Eur J Histochem 2009;53:113-6. [Crossref] [PubMed]
Cite this article as: Lau J, Guminski A, Gill A, Veivers D. Metastatic basal cell carcinoma: a review of six cases. Aust J Otolaryngol 2018;1:20.


