
Inner ear patency after retrosigmoid vestibular schwannoma resection
Introduction
Vestibular schwannomas (VS) are benign nerve sheath tumors arising from Schwann cells of the vestibulocochlear nerve (1). They present with a range of symptoms from vestibulocochlear nerve dysfunction to mass effect in the cerebellar pontine angle (2). Surgical intervention remains an important treatment modality (3) and common surgical approaches include resection via a translabyrinthine (TL) (4) or retrosigmoid (RS) (5) craniotomy.
The RS craniotomy represents a versatile technique that allows for resection of most VS, provides excellent exposure of the brainstem and is commonly used when hearing preservation is a primary aim of surgery by reducing the risk of damage to the vestibular and cochlear labyrinth (5). Despite this, unilateral hearing loss subsequent to RS VS resection remains a significant issue. Reported short term hearing preservation rates range from 15–74.1% (6-8) and long term preserved hearing is lower with significant incidence of accelerated deterioration in longer term post-operative outcomes (9). Unilateral hearing loss causes significant disability in communication and social interactions, particularly in background noise and difficult acoustic conditions (10).
Mechanisms for hearing loss during VS resection include direct damage to the cochlear nerve, otic capsule, cochlea, vestibule, or their vascular supply (11). In cases where hearing loss has occurred despite an apparently intact cochlear nerve, evidence suggests cochlear implantation (CI) can be useful to alleviate the burden of unilateral hearing loss (12).
A potential surgical challenge for CI post VS resection is the possible ossification or fibrosis of the cochlea (12) which may occur due to surgical trauma or resulting inflammation (13). Vascular injury, specifically to the labyrinthine artery, has also been identified as a cause of progressive fibrosis and ossification of the cochlea (14,15). Currently limited published research exists identifying the patterns of fibrosis or ossification following RS VS resection with only one published paper examining cochlear obliteration following surgery (16). T2 weighted magnetic resonance imaging (MRI) of the inner ear has been shown to correlate well with operative findings of cochlear obstruction due to fibrosis or ossification (17). The aim of this study is to further evaluate inner ear patency and fibrosis following RS VS resection using post-operative T2 MRI scans to further inform the potential use and timing of CI to alleviate unilateral hearing loss in this patient population.
Methods
Ethical approval (HREC/17/MH/363) was gained to identify and review records of patients who had underwent VS resection by a RS craniotomy over a 4-year period from 2011 to 2014 at a single institution (1). Thirty-five patients were identified. Four patients were excluded: two had limited post-operative imaging available for review, one subsequently underwent a TL VS resection for recurrence, and one required further temporal bone surgery for a cerebrospinal fluid (CSF) leak. All patients had received regular clinical review including post-operative MRI scans. Scans were performed between 2 to 6 months post operatively, then approximately every twelve months thereafter. Scans with 0.6 to 0.8 mm slice thickness were reviewed and all scans were performed on 1.5- or 3-Tesla unit.
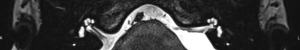
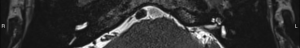
For this study the preoperative and post-operative MRI scans were retrospectively reviewed. Tumour size and volume using the ABC/2 method was measured by reviewing preoperative MRI scans. The first, second and most recent post-operative MRI scans were subsequently reviewed. Both vestibular and cochlear labyrinth fluid signals in the operated ear were evaluated using T2 weighted MRI scans. The vestibular labyrinth was graded to be either: normal (no evidence of fluid signal change, e.g., Figure 1), or abnormal (evidence of fluid signal change, e.g., Figure 2). The cochlear labyrinth was graded into four groups:
- Grade 0: a cochlea without evidence of any signal changes (e.g., Figure 3);
- Grade 1: a cochlea with a minor hypointense signal throughout and/or loss of signal in only one scala (e.g., Figure 4);
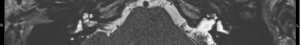
- Grade 2: partial obliteration of the whole cochlea with at least 20% of the cochlea lumen still having normal signal (e.g. Figure 5);
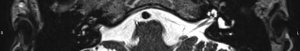
- Grade 3: complete obliteration of the cochlea as defined as less than 20% of the cochlea having a fluid signal (e.g. Figure 6).
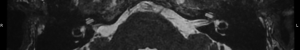
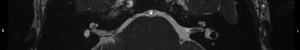
Review and grading of the patient’s MRI scans were independently performed by two Otolaryngologists (Fiona C. E. Hill, Shannon Withers) comparing the operated inner ear with the contralateral ear. A weighted Kappa score was calculated to determine inter rater reliability. Where a difference in grading opinion occurred, the scans were re-reviewed and an agreed score used. When inner ear fluid signal changes were evident on the first post-operative scan, the distance between the posterior semicircular canal and the CSF was measured to determine likelihood of potential otic capsule breach. Where doubt existed post-operative CT brain scans were reviewed to further evaluate the otic capsule.
Imaging results were cross referenced with pre and post-operative audiograms that were performed approximately three months post-surgery. Thresholds in the affected ears were recorded at 500, 1,000, 2,000 and 4,000 Hz. Hearing outcomes where defined as preserved, serviceable or no recordable hearing. Preserved hearing was defined as any measurable hearing post-surgery. Serviceable hearing was defined as air conduction pure tone average of 500 Hz, 1, 2 and 4 kHz less than 50 dB. Where audiograms were not available hearing outcomes were investigated by reviewing post-operative correspondence, such as outpatient clinical letters, where clear hearing outcomes were described.
Results
Eighty-five MRI scans of 31 patients were reviewed to assess the time course of vestibular and cochlear labyrinth fluid signal changes post RS VS resection. The characteristics of patients are listed in Table 1.
Table 1
| Characteristics | Number |
|---|---|
| Male/female (n) | 15/16 |
| Age range (year) | 32–65 |
| Average age (year) | 51 |
| Right side/left side (n) | 22/9 |
| Average tumour dimensions (width × length × height) (mm3) | 19×14×14 |
| Average tumour volume (cm3) | 3.24 |
| Tumour volume range (cm3) | 0.08–18.45 |
The first post-operative MRI scan was performed between 2 to 12 months (58 to 378 days) with an average of approximately 5 months (151 days). Fourteen patients were identified to have a normal vestibular and cochlear fluid signal on their first scan (45%). All remaining patients exhibited an abnormal vestibular labyrinth signal. Of these 7 had a grade 0 cochlea (23%), 4 a grade 1 cochlea (13%), 5 a grade 2 cochlea (16%) and 1 a grade 3 cochlea (3%).
The second post-operative scan was performed between 12 to 41 months (352 to 1,235 days) at an average of 18 months (545 days). Again 14 patients had a normal vestibular labyrinth signal and grade 0 cochlea (45%). The number of patients with an abnormal vestibular labyrinth signal and a grade 0 cochlea remained unchanged (23%) as did patients with a grade 2 cochlea (16%). The number of patients with an abnormal labyrinth and a grade 1 cochlea decreased to 2 (6%) and those with a grade 3 cochlea increased to 3 (10%).
The most recent post-operative scan was performed between 21 to 76 months (636 to 2,304 days) with an average approximately 44 months (1,344 days). Again, there was no change in the number of patients with normal vestibular labyrinth signal and a grade 0 cochlea (45%) or abnormal vestibular labyrinth with a grade 0 (23%) or grade 2 (16%) cochlea. The number of patients with a grade 1 cochlea again decreased to 1 (3%) while those with a grade 3 cochlea increased to 4 (13%).
A weighted Kappa score was calculator to look for inter rater reliability. For the assessment of cochlear fluid signal the weighted Kappa was 0.949, with a standard error of 0.041 and a 95% confidence interval from 0.846 to 1.00. For the assessment of the vestibular labyrinth fluid signal the weighted Kappa was 1. This placed the inter-rater reliability into the top category of “very good” for both Kappa scores.
Progression of fluid signal loss was only ever identified in patients with cochlear fluid signal changes on their first post-operative scan and only in a sequential manner. Two patients with grade 1 cochlear fluid signal changes progressed to grade 2 changes (50%), and two patients with grade 2 cochlear fluid changes progressed to grade 3 changes (40%) on their second scan. Two different patients progressed between their second and most recent scan, with one progressing from grade 1 to 2 changes and another from grade 2 to 3 changes. All results are summarized in Table 2.
Table 2
| Grading | Number on 1st scan | Number on 2nd scan | Number on latest scan |
|---|---|---|---|
| Normal vestibular labyrinth | |||
| Grade 0 cochlea | 14 (45%) | 14 (45%) | 14 (45%) |
| Abnormal vestibular labyrinth | |||
| Grade 0 cochlea | 7 (23%) | 7 (23%) | 7 (23%) |
| Grade 1 cochlea | 4 (13%) | 2 (6%) | 1 (3%) |
| Grade 2 cochlea | 5 (16%) | 5 (16%) | 5 (16%) |
| Grade 3 cochlea | 1 (3%) | 3 (10%) | 4 (13%) |
| Total | 31 | 31 | 31 |
Of the 17 patients with inner ear fluid signal changes none had convincing overlap of their posterior semicircular canal and the CSF signal. The distance between these signals ranges from 1 to 7 mm with an average distance of 3.5 mm. Three patients were considered to be indeterminate due to proximity or clarity of imaging. Review of post-operative CT brain scans were not suggestive of breach of the otic capsule.
The average tumour volume was 3.24 cm3 with a range of 0.08 to 18.45 cm3. No significant difference in tumour volume means for normal and abnormal vestibular labyrinth fluid signal status on the first post-operative MRI scan was identified using a two-sample t-test (t=1.76, df =22, P=0.09). Likewise no significant difference in tumour volume means for cochlear fluid signal grade on the first post-operative MRI scan was identified using a one factor ANOVA [F(3,24)=0.06, P=0.6].
Thirty-one pre-operative and 23 post-operative audiograms were available for review. Pre-operative audiograms occurred between 1 to 311 days with an average of 75 days prior to surgery. All but one pre-operative audiograms demonstrated measurable hearing on the tumour side with air conduction thresholds at an average of 17, 22, 31 and 42 dB at 500 Hz, 1, 2 and 4 kHz respectively. Post-operative audiograms occurred between 13 to 360 days, with an average of 103 days post-surgery. Results are summarized in Table 3. Of the 8 individuals without post-operative audiograms available for review 6 had correspondence revealing hearing outcomes, with all indicating that the patient had lost all hearing in the operated ear.
Table 3
| Audiograms | Number (n) | Average 500 Hz (dB) | Average 1 kHz (dB) | Average 2 kHz (dB) | Average 4kHz (dB) |
|---|---|---|---|---|---|
| Pre-operative audiograms | 31/31 | 17 | 22 | 31 | 42 |
| Post-operative audiograms with recordable response | 10/23 | 32 | 34 | 42 | 64 |
| Post-operative audiograms with no recordable response (at all frequencies) | 13/23 | – | – | – | – |
Of the 29 patients with identifiable outcome, measurable hearing was preserved in 9 patients (31%) of which 6 had serviceable hearing (21%). All had a normal fluid signal in the whole of the labyrinth. Of these patients 6 had serviceable hearing (21%). No recordable audiological response was confirmed by audiogram in 14 patients and by correspondence for a further 6 patients (69%). Of the 13 patients with a normal fluid signal in the whole labyrinth 4 had no recordable hearing (31%). All 16 patients with an abnormal signal in any part of the vestibular or cochlear labyrinth, had no recordable hearing. Two patients without identifiable hearing outcomes where excluded from their category in the correlation between grading and hearing outcomes. Patients with preserved hearing had significantly smaller tumour volumes (0.55 cm3) compared to those with no recordable hearing (4.29 cm3) using a two-sample t-test (t=3.08, df =22, P=0.005). Results are summarized in Table 4.
Table 4
| Grading | Number of patients with preserved hearing | Number of patients with no audiological response |
|---|---|---|
| Normal vestibular labyrinth | ||
| Grade 0 cochlea | 9 (31%) | 4 (14%) |
| Abnormal vestibular labyrinth | ||
| Grade 0 cochlea | 0 | 7 (24%) |
| Grade 1 cochlea | 0 | 4 (14%) |
| Grade 2 cochlea | 0 | 4 (14%) |
| Grade 3 cochlea | 0 | 1 (3%) |
| Total | 9 (31%) | 20 (69%) |
Discussion
While a RS approach to VS resection may be intended as a hearing preservation technique, hearing loss remains one of the most common complications with this study demonstrating a hearing preservation rate of 31%. Options for aural rehabilitation after unilateral hearing loss include amplification techniques to route sound to the contralateral unaffected ear such as with a contralateral routing of sound hearing aid (CROS) or bone anchored hearing aid (BAHA). However, CI has the potential to provide binaural hearing benefits for unilateral hearing loss. Cochlear implants have been found to offer improved speech in noise performance, sound localization, tinnitus perception and quality of life over CROS aids and BAHA (18). CI has been successfully performed in individuals post RS VS resection in order to help with hearing loss (19-21). Successful implantation requires both an intact cochlear nerve and a patent cochlea. Cochlear patency can be affected by fibrosis and ossification post VS resection (12).
No accepted methodology for grading of the vestibular or cochlear labyrinth fluid signal changes post VS resection on T2 MRI has been reported in the literature. The authors have proposed a pragmatic grading system that reflects the anatomical factors which impact upon CI surgery. A cochlea without any fluid signal changes on MRI represents the ideal clinical presentation (grade 0). A cochlea with a hypointense fluid signal throughout or only loss of signal in one scala represents the next preferred clinical presentation (grade 1). While a cochlea with almost complete loss of signal (grade 2) or an obliterated cochlea (grade 3) represents an unfavorable clinical presentation with the lowest chance of successful electrode insertion.
Of the patients with an abnormal signal at any part of the vestibular or cochlear labyrinth, 16 had no recordable hearing (100%). Preserved hearing was not guaranteed by a normal inner ear fluid signal on post-operative MRI. Of patients with a normal vestibular labyrinth and grade 0 cochlea on their first MRI scan 4 had no recordable hearing post operatively (31%). While this may represent sacrifice of or damage to the cochlear nerve, review of the operative reports of these individuals indicate the surgical team reported the cochlear nerve to be intact. Damage to the labyrinthine artery has been shown to result in complete hearing loss (11) and may account for this finding. Inadvertent breach of the otic capsule may also explain this result, however review of imaging revealed no individual exhibited convincing evidence of this. It is worth noting that the senior surgeon utilizes a relatively conservative transmeatal approach, avoiding excessive lateral bone removal. Tumour removal from the lateral internal auditory canal is achieved with endoscopic assistance and an angled dissector. The authors are confident that this technique does not lead to higher rates of residual tumour or greater risk of damage to the labyrinthine artery.
All patients that had a normal cochlea on their first MRI scan regardless of their vestibular labyrinth status maintained a normal cochlear fluid signal on their subsequent scans. One individual maintained a grade 0 cochlea for 6 years post-surgery. Thus, timing to implantation in these individuals is not necessarily critical from an obliteration point of view. Indeed, at least one case report documents successful CI three years after RS VS resection (19). Although a concern for CI post VS resection is the impact on the ability to perform surveillance MRI scans and adequately visualize the internal auditory canal (22).
Timing of CI surgery is potentially more critical for those with evidence of cochlear fluid signal change on their first post-operative scan. Thirty-two percent of patients had evidence of vestibular and cochlear labyrinth fluid signal change on their first post-operative scan and a significant proportion of these showed progression on subsequent scans. Cochlear fluid signal change on MRI as an indication of fibrosis has been identified as a factor for difficult CI post bacterial meningitis. However full electrode insertion was still achieved in many cases despite partial cochlear obstruction (23). This finding suggests that successful implantation in patients with a grade 1, 2 or 3 cochlea is less likely compared to a grade 0 cochlea, but may still be attempted.
The results of this study suggest that when CI may be considered post RS VS resection, in patients with no serviceable hearing and a preserved cochlear nerve, early post-operative MRI is useful to evaluate inner ear fluid signal change. This will allow prediction and timing of successful electrode insertion. Neurophysiological testing may also be useful prior to implantation to demonstrate an intact cochlear nerve (20).
Another potential consideration is for CI at time of RS VS resection. Given a significant number of patients had fluid signal changes on their first scan (32%) CI may be better performed at the time of surgery to avoid loss of cochlear patency. This represents a dilemma to either try and identify patients who would benefit from CI at time of surgery versus waiting to identify post-operative outcomes in the knowledge some patients will lose cochlear patency. Methods such as intraoperative auditory brainstem testing (24) and cochlear promontory stimulation (20) have been successfully used to monitor cochlear nerve function with simultaneous CI post VS resection.
Cochlear patency could be maintained by implantation of a placeholder electrode at the time of initial surgery. Placeholder electrodes have successfully maintained cochlear patency after TL VS resection for later CI implantation (12). However, this is not as practical following a RS VS resection as the cochlear has not been surgically exposed. Further the hearing outcome is not immediately evident during surgery and placement of such an electrode risks compromising preserved hearing.
Tumor volume was not found to be significantly different between patients with or without vestibular labyrinth or cochlear fluid signal changes. However, tumour volume was significantly smaller in those patients who had preserved hearing. This is in keeping with previous findings that tumour size is a predictor of hearing preservation, with tumours less than 1 cm in size providing the best chance of preservation (25,26).
The results of this study can be compared with patients who have undergone TL VS resection. The authors have found that 82% of these patients will have cochlear fluid signal changes after 6 months and the majority will progress to more severe fluid signal changes over 48 months (27). Given that hearing loss post TL VS resection is inevitable (4) the authors have suggested that where possible CI should take place either at the time of surgery or as soon as possible to maximize the change of successful implantation (27). Comparatively for patients undergoing RS VS resection, hearing outcomes are unknown and the majority of patients will have a patent cochlear. As such consideration of CI should occur as a second stage procedure, although delay beyond 6 months may result in patient’s losing cochlear patency through fibrosis or ossification.
This study represents a detailed assessment of the patterns of vestibular and cochlear labyrinth fluid signal change correlated with hearing outcomes post RS VS resection. It also provides some insight into the cause of hearing loss post RS VS resection. Further work is needed to better understand the pathogenesis of hearing loss in this patient population in an attempt to improve preservation rates and identify factors that lead to successful CI in this group.
Conclusions
The majority of patients that underwent RS VS resection maintained a patent cochlea many years after surgery. A significant number of patients who had evidence of cochlear fluid signal change on their first post-operative scan went on to develop increased loss of fluid signal on subsequent imaging. Ninety percent of patients with loss of signal on MRI had no detectable hearing on audiometry. These findings suggest that patients are at risk of fibrosis of the cochlear lumen after RS VS resection. Individuals who may be considered for CI and have early signal changes on post-operative MRI should ideally undergo implantation as soon as possible to maximize the chance of successful implantation.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.09.07). CI serves as an unpaid editorial board member of Australian Journal of Otolaryngology. RB serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval was obtained from the Melbourne Health Human Research Ethics Committee (HREC/17/MH/363), as this was a low risk quality assurance study using information from health records, an exemption from informed consent was sort and granted from the Melbourne Health Human Research Ethics Committee. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pytel P, Anthony D. Peripheral Nerves and Skeletal Muscle. In: Kumar V, Abbas A, Aster J. editors. Robbins and Cotran Pathologic Basis of Disease. Ninth ed. Philadelphia, PA: Elsevier/Saunders, 2015:1246-7.
- DeLong M, Kaylie D, Kranz P, et al. Vestibular Schwannomas: Lessons for the Neurosurgeon: Part I: Diagnosis, Neuroimaging, and Audiology. Contemp Neurosurg 2011;33:1-5. [Crossref]
- Arthurs B, Fairbanks R, Demakas J, et al. A review of treatment modalities for vestibular schwannoma. Neurosurg Rev 2011;34:265-77. [Crossref] [PubMed]
- Arriaga MA, Lin J. Translabyrinthine approach: indications, techniques, and results. Otolaryngol Clin North Am 2012;45:399-415. [Crossref] [PubMed]
- Elhammady M, Telischi F, Morcos J. Retrosigmoid approach: indications, techniques, and results. Otolaryngol Clin North Am 2012;45:375-97. [Crossref] [PubMed]
- Harner S, Beatty C, Ebersold M. Retrosigmoid removal of acoustic neuroma: experience 1978-1988. Otolaryngol Head Neck Surg 1990;103:40-5. [Crossref] [PubMed]
- Holsinger F. Hearing preservation in conservation surgery for vestibular schwannoma. Am J Otol 2000;21:695-700. [PubMed]
- Nonaka Y, Fukushima T, Watanabe K, et al. Contemporary surgical management of vestibular schwannomas: analysis of complications and lessons learned over the past decade. Neurosurgery 2013;72:103-15. [PubMed]
- Chee G, Nedzelski J, Rowed D. Acoustic Neuroma Surgery: The Results of Long-term Hearing Preservation. Otol Neurotol 2003;24:672-6. [Crossref] [PubMed]
- Wie O, Pripp A, Tvete O. Unilateral deafness in adults: effects on communication and social interaction. Ann Otol Rhinol Laryngol 2010;119:772-81. [PubMed]
- Babbage M, Feldman M, O'Beirne G, et al. Patterns of Hearing Loss Following Retrosigmoid Excision of Unilateral Vestibular Schwannoma. J Neurol Surg B Skull Base 2013;74:166-75. [Crossref] [PubMed]
- Hassepass F, Arndt S, Aschendorff A, et al. Cochlear implantation for hearing rehabilitation in single-sided deafness after translabyrinthine vestibular schwannoma surgery. Eur Arch Otorhinolaryngol 2016;273:2373-83. [Crossref] [PubMed]
- deSouza C, Paparella MM, Schachern P, et al. Pathology of labyrinthine ossification. The J Laryngol Otol 1991;105:621-4. [Crossref] [PubMed]
- Perlman HB, Kimura R, Fernandez C. Experiments on temoporary obstruction of the internal auditory artery. Laryngoscope 1959;69:591-613. [Crossref] [PubMed]
- Belal A. Pathology of vascular sensorineural hearing impairment. Laryngoscope 1980;90:1831-9. [Crossref] [PubMed]
- Hedjrat A, Schwager K, Hofmann E, et al. Postoperative Cochlear Obliteration after Retrosigmoid Approach in Patients with Vestibular Schwannoma. J Neurol Surg B Skull Base 2017;79:343-8. [PubMed]
- Isaacson B, Booth T, Kutz JW, et al. Labyrinthitis ossificans: How accurate is MRI in predicting cochlear obstruction? Otolaryngol Head Neck Surg 2009;140:692-6. [Crossref] [PubMed]
- Arndt S, Aschendorff A, Laszig R, et al. Comparison of Pseudobinaural Hearing to Real Binaural Hearing Rehabilitation After Cochlear Implantation in Patients With Unilateral Deafness and Tinnitus. Otol Neurotol 2011;32:39-47. [Crossref] [PubMed]
- Dagna F, Murri A, Albera R, et al. Cochlear implantation in delayed sudden hearing loss after conservative vestibular schwannoma surgery. Acta Otorhinolaryngologica Italica 2016;36:428-30. [PubMed]
- Ferreira Bento R, Alves Monteiro T, Gomes Bittencourt A, et al. Retrolabyrinthine approach for cochlear nerve preservation in neurofibromatosis type 2 and simultaneous cochlear implantation. Int Arch Otorhinolaryngol 2013;17:351-5. [Crossref] [PubMed]
- Çelenk F, Cevizci R, Altınyay S, et al. Cochlear Implantation in Extraordinary Cases. Balkan Med J 2015;32:208-13. [Crossref] [PubMed]
- Crane B, Gottschalk B, Niparko J, et al. Magnetic Resonance Imaging at 1.5 T After Cochlear Implantation. Otol Neurotol 2010;31:1215-20. [Crossref] [PubMed]
- Nichani J, Green K, Hans P, et al. Cochlear implantation after bacterial meningitis in children: outcomes in ossified and nonossified cochleas. Otol Neurotol 2011;32:784-9. [Crossref] [PubMed]
- Hummel M, Perez J, Hagen R, et al. Auditory Monitoring in Vestibular Schwannoma Surgery: Intraoperative Development and Outcome. World Neurosurg 2016;96:444-53. [Crossref] [PubMed]
- Jacob A, Robinson LL Jr, Bortman JS, et al. Nerve of origin, tumor size, hearing preservation, and facial nerve outcomes in 359 vestibular schwannoma resections at a tertiary care academic center. Laryngoscope 2007;17:2087-92. [Crossref] [PubMed]
- Mislav G, Milan R. What Is the Best Tumor Size to Achieve Optimal Functional Results in Vestibular Schwannoma Surgery? Skull Base An Interdisciplinary Approach 2008;18:317-25.
- Hill FCE, Grenness A, Withers S, et al. Cochlear Patency After Translabyrinthine Vestibular Schwannoma Surgery. Otol Neurotol 2018;39:575-9. [Crossref] [PubMed]
Cite this article as: Grenness A, Hill FCE, Withers S, Iseli C, Briggs R. Inner ear patency after retrosigmoid vestibular schwannoma resection. Aust J Otolaryngol 2018;1:26.


