
The use of THRIVE in otolaryngology: our experiences in two Australian tertiary facilities
Introduction
THRIVE (transnasal humidified rapid-insufflation ventilatory exchange) is the delivery of transnasal humidified oxygen by high-flow nasal prongs.
The circuit consists of an air-oxygen blender attached to a flow meter, which controls the concentration and flow of oxygen delivered. This is connected to a heated humidifier which delivers warmed, humidified oxygen through high-performance tubing to wide-bore nasal prongs, designed to fit snugly at the entrance to the nares. It can generate up to 100% FiO2 at 70 L/min, with a positive end-expiratory pressure of 7.4 cmH2O.
High-flow nasal oxygen has a well-established use in critical care and ICU settings as a method of providing high volumes of oxygen, typically in patients with respiratory failure. However, it has also seen use as an adjunct in difficult intubations as a method for pre-oxygenation and apnoeic oxygenation (1). The concept revolves around extension of the apnoeic window—the small timeframe following anaesthetic induction for securing a definitive airway. Traditionally, pre-oxygenation has been performed by facemask ventilation with 100% oxygen, the aim of which is to displace nitrogen in the lungs and create an oxygen reservoir. As long as a patent airway exists, the difference in rate of alveolar oxygen removal and carbon dioxide excretion generates a negative pressure gradient that pulls oxygen into the lungs. This phenomenon is known as aventilatory mass flow (2). The application of a high-flow nasal delivery system such as Optiflow (Fisher & Paykel Healthcare, New Zealand) aims to provide large volumes of oxygen to the lungs to create an oxygen reservoir, prevent entrainment of room air to ensure continual delivery of a high concentration of oxygen, and reduce the rate of build-up of carbon dioxide.
Patel and Nouraei [2015] trialled THRIVE for pre-oxygenation in 25 patients with known difficult airways, and found a mean apnoea time of 17 min with no desaturations below 90%. Furthermore, two patients received THRIVE for the duration of their procedures, with apnoea times of 32 and 65 min (2).
Maupeu et al. (3) conducted a prospective study of 19 patients where THRIVE was utilized for suspension microlaryngoscopy cases, and found a mean apnoea time of 27 min. Two cases were noted where THRIVE was unable to be utilized effectively due to morbid obesity (BMI >35).
To et al. (4) performed 17 cases of balloon dilatation of subglottic stenosis utilizing THRIVE and found a median apnoea time of 18 min. Two cases required bag-mask-ventilation for desaturation during THRIVE.
Up until now, THRIVE has predominantly been an anaesthetic adjunct for difficult airways. However, there is evidence to suggest that it may be used as the sole method of ventilation in short phonolaryngeal procedures (5).
Methods
We describe our experiences with THRIVE in otolaryngology since the start of 2017 at two tertiary facilities in Sydney: Westmead Public Hospital/Westmead Private Hospital and Chris O’Brien Lifehouse. Twenty-eight cases were retrospectively reviewed where THRIVE was used in phonolaryngeal surgery with the support of an experienced anaesthetist (Murray Stokan). Cases included operations on supraglottic, glottic and subglottic pathology, with some involving the CO2 laser.
Our protocol for THRIVE included using an Optiflow THRIVE unit with optional blender. This circuit consisted of an air/oxygen blender and flow meter, heated humidifier, and wide-bore nasal prongs.
Pre-oxygenation was conducted via the application of nasal prongs prior to induction at 30 L/min at FiO2 100% for a minimum of 3 min.
Anaesthetic induction utilized midazolam, propofol target controlled infusions (TCI) and remifentanil TCI. Muscle relaxant (rocuronium) was given at induction to allow the peak effect to be reached before laryngoscopy. Following induction, the flow rate of THRIVE was increased to between 50–70 L/min.
Jet ventilation supplementation was considered if oxygen saturations dropped under 90%. This was context specific, however. For example, in the stable patient, sub-90% saturations were tolerated if it occurred at the time of specimen removal, to avoid jetting the specimen into the airway. If frequent jet ventilation was required, reversion to intubation with an endotracheal tube (ETT) was considered.
For procedures involving the use of laser, standard laser safety precautions and contingency plans were observed. This included the presence of a laser safety officer in the operating theatre, eye protection for operating staff, wet gauze draped over the patient’s eyes, containers of water present in case of a fire, and confirmation of correct functioning prior to commencement of case. Particular caution was observed to avoid pooling of oxygen and gases underneath the drapes. A forced air warmer was used to ventilate beneath the drapes, and head drapes were checked to ensure they were not covering the nasal prongs. Our laser protocol was to drop the THRIVE FiO2 to 30% prior to commencement of laser, followed by suctioning out of airway gases.
THRIVE was only trialled in cases where the expected duration of phonolaryngeal/trachea-bronchial surgery was less than 60 min. There was no exclusion based on BMI, but note was made of potential challenges when using THRIVE in obese patients due to lower lung reserve (functional residual capacity) and increased incidence of OSA. There was no change in THRIVE protocol for obese patients. THRIVE was not used in patients with GORD due to risk of gastric aspiration.
Results
Figure 1 demonstrates our setup and approach to using THRIVE in otolaryngology.

Table 1 illustrates the range of phonolaryngeal/tracheobronchial cases where we have trialled THRIVE as the sole method of ventilation for the duration of procedure. Included amongst the twenty-eight cases are operations for complex airways, microlaryngoscopies +/− bronchoscopies and laser cases (Figure 2).
Table 1
| Operation | No. of patients |
|---|---|
| Difficult intubations | |
| Tracheostomy under local anaesthetic for supraglottic mass | 3 |
| Awake fibreoptic intubation for epiglottic abscess | 1 |
| Microlaryngoscopy | |
| Laryngotracheobronchoscopy | 3 |
| Microlaryngoscopy + coblation of lesion | 2 |
| Microlaryngoscopy + biopsy (including biopsy of epiglottis) | 3 |
| Microlaryngoscopy + vocal cord resection | 1 |
| Rigid ventilating bronchoscopy + biopsy of bronchogenic tumour | 1 |
| External injection thyroplasty under local/sedation | 4 |
| Laser | |
| Laser of vocal cord lesion | 6 |
| Laser & dilatation of subglottic stenosis | 3 |
| Laser cordotomy | 1 |

Within this retrospective review of 28 patients, none required intubation or oral airway adjuncts/bag-mask ventilation mid-procedure.
Figure 3 details the oxygen saturation trend for a 41-year-old gentleman who underwent an elective laser resection of vocal cord lesion for whom THRIVE was utilized. THRIVE was paused intermittently whilst CO2 laser was in use, with jet ventilation supplementation implemented when saturations dropped to 90%. This method was successful for the entirety of the 26 min case.
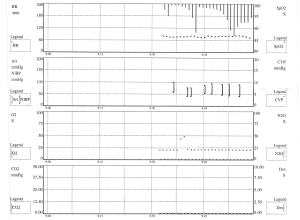
THRIVE was also trialled in a 45-year-old lady who underwent an elective microlaryngoscopy with laser and dilatation of a 5 mm subglottic stenosis. THRIVE was paused briefly whilst CO2 laser was used to create radial cuts in the subglottic stenosis. As the periods of laser use were short, and quickly followed by re-commencement of 100% THRIVE, jet ventilation was unnecessary and no desaturations under 90% were observed (Figure 4).
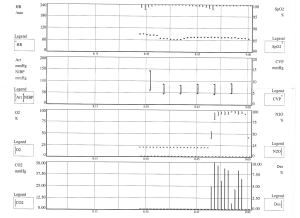
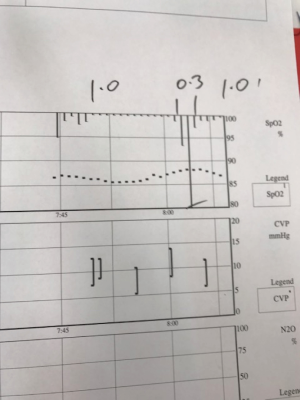
THRIVE was similarly trialled in a 38-year-old lady with a BMI of 43 undergoing an elective microlaryngoscopy with laser and dilatation of an 8 mm subglottic stenosis (Figure 5). THRIVE was initially successful in maintaining saturations when the FiO2 was 1.0, but when the FiO2 was dropped to 0.3 using the blender for laser commencement, desaturation under 90% occurred. This rapidly corrected with the implementation of jet ventilation. This technique was effective for the entirety of the 23 min case (Figure 6).
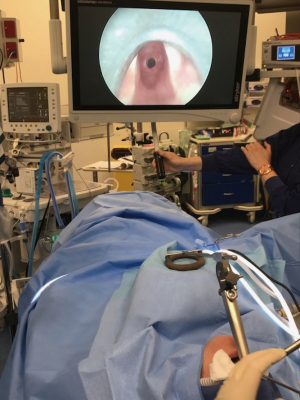
We have also found THRIVE to be useful in semi-emergent/emergent settings, as a method of pre-oxygenation and for maintenance of oxygen saturations in cases where a definitive airway must be secured.
In one such case, THRIVE was used for oxygenation whilst performing an awake tracheostomy under local anaesthetic for a gentleman with a T4a supraglottic tumour (Figure 7). He maintained saturations >90% throughout.
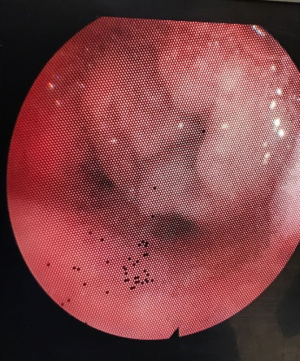
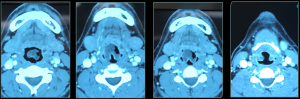
Figure 8 demonstrates the radiological findings of a patient who presented to emergency with an epiglottic abscess. This patient required emergent awake fibreoptic intubation. Figure 9 demonstrates successful intubation past the epiglottic abscess, with THRIVE used as an adjunct to maintain oxygen saturations throughout the intubation period.

Discussion
Our preliminary trials of THRIVE in phonolaryngeal surgery and as an adjunct in emergent airways have shown it to be safe and effective for procedures of short duration.
One advantage of THRIVE is that it prolongs the apnoeic window when used for pre-oxygenation, allowing for more time to secure a definitive airway, reducing stress in complex scenarios. It also offers an atraumatic alternative to endotracheal intubation in suitable patients, avoiding complications such as laryngeal trauma, laryngospasm, vocal cord palsy etc. For laryngeal operations, it allows for an improved field of view as there is no obstructing ETT. This facilitates diagnostic assessment and allows for accurate grading of laryngeal view, important when selecting appropriate cases for transoral laser surgery. In some cases, it may even allow airway procedures of higher complexity to be performed due to ease of access to the relevant site. As high-flow nasal oxygen has already had a well-established use in critical care units, many anaesthetic departments are familiar with its setup and use, allowing for smooth transition into the operative setting. The current alternative to endotracheal intubation in laryngeal procedures is jet ventilation. This involves providing intermittent short bursts of oxygen delivery to the airway. Compared to jet ventilation, THRIVE does not cause vibration of tissue. This is particularly relevant in microsurgical work where surgery must pause whilst jet ventilation is performed. THRIVE thus has the advantage of improved efficiency and smoother operating.
Different protocols for THRIVE have been described. For example, Patel & Nouraei [2015] (2) described a technique of pre-oxygenating at 40 degrees head-up with 70 L/min for 10 min. In our practice, we have found that a flow rate of 30 L/min is better tolerated when the patient is awake, which can then be increased to 70 L/min post-induction. We also advocate for administration of muscle relaxant at time of induction to allow for peak effect to be reached at time of laryngoscopy to minimise trauma to tissues. Potential complications that may arise from using THRIVE include epistaxis (risk reduced with humidification and warming of air), local discomfort, gastric distension as well as potential aspiration of blood and gastric contents. The effective FiO2, PEEP and flow rates are affected by the fit of nasal cannula and whether the patient has their mouth open—as such, the actual amount and rate of oxygen delivery may differ from that proposed.
THRIVE is dependent on a clear nasal passage to facilitate high flows, and thus should not be used in patients with severe nasal obstruction. It should be avoided in patients with suspected or confirmed pneumothorax, as well as those with bullous lung disease. THRIVE is also contraindicated in patients with base of skull fractures or mid maxillary facial trauma due to the risk of pneumocephalus, and subcutaneous emphysema respectively.
Our observations in patients with OSA were that THRIVE could still be effective if the airway was well supported. This involved a short induction to laryngoscopy time, oropharyngeal airway, jaw support, and keeping the mouth closed to maintain positive airway pressure and applying intermittent bag-mask ventilation until suspension laryngoscopy could be setup to hold the airway open.
In the absence of a cuffed ETT, there is a theoretical risk of macro-aspiration of blood and laryngeal contents. This was prevented by supine positioning, meticulous haemostasis with adrenaline-soaked gauze pads, and frequent suctioning of airway secretions and blood. At the conclusion of a case, the airway was inspected down to the level of the carina and proximal main bronchi. If there was concern regarding gross aspiration, flexible bronchoscopy was considered. No patient required this in the cases reviewed.
Controversy currently surrounds the concurrent use of THRIVE and the CO2 laser due to a theoretical risk of airway fires. In our practice, we have not trialled the use of laser concurrent with THRIVE (THRIVE is paused, making sure that any potential O2 pooling in the airway has been suctioned and replaced with jet ventilation when needed for the duration of laser use). It is important to emphasise that airway fires require three components: fuel/oxidizer, ignition (heat) and oxygen. Although an ignition source and oxygen are present when THRIVE is used in laser surgery, there is no combustible material or fuel to facilitate an airway fire. ETTs constructed from polyvinylchloride can potentially act as fuel for airway fires—this risk is reduced through the use of laser-resistant ETTs for routine laser cases. However, in the context of THRIVE, this is obsolete as an ETT is not required at all. Gauze strips can also act as potential fuel; care should be taken to ensure no foreign material such as neuro patties remain in the airway at the time of laser. Onwochei et al. (8) reported a case of intra-oral ignition of monopolar diathermy, whereby an arc between the diathermy tip and a titanium dental implant resulted in brief ignition of the polytetrafluroethylene grip on the diathermy shaft. This case reinforces the importance of being aware of the “fire triad” and addressing each component to minimize the chance of these dangerous, albeit rare, events. Strategies to reduce the risk of airway fires when using THRIVE in laser surgery include: ensuring removal of all foreign material from the airway prior to laser, reducing the FiO2 prior to laser commencement (using the air/oxygen blender), stopping THRIVE during laser and scavenging any residual oxygen within the airway +/− supplementation with jet ventilation between laser periods.
It should be noted that even if the fuel within the airway is removed, the patient’s hair, drapes, monitors, thrive circuit, bedding etc. are all fuels and are bathed in the oxygen plume that surrounds the patient’s head. With high flows of oxygen passing through the circuit at up to 70 L/min, much of this will pass back out into the patient surroundings. As such, the greatest risk of fire to the patient is arguably ignition of the patient’s surrounds, as opposed to airway material.
An interesting concept is the use of THRIVE in paediatric patients. There have been varying results whilst using THRIVE for pre-oxygenation in children. A randomised controlled trial of 48 patients by Humphreys et al. (9) demonstrated that THRIVE prolongs the safe apnoea time in healthy children but has no effect to improve carbon dioxide clearance. Conversely, a randomised controlled trial in 60 paediatric patients by Riva et al. (10) showed that THRIVE did not extend the safe apnoea time. More studies are required in this area.
Conclusions
We advocate for the continued trialling of THRIVE as the sole method of ventilation in patients undergoing short phonolaryngeal/tracheobronchial procedures.
Acknowledgments
Presented at the Australian and New Zealand Head & Neck Cancer Society Annual Scientific Meeting as an oral presentation [2017].
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.07.02). FR serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained for the publishing of all images and videos in this paper.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Binks MJ, Holyoak RS, Melhuish TM, et al. Apnoeic oxygenation during intubation in the intensive care unit: A systematic review and meta-analysis. Heart Lung 2017;46:452-7. [Crossref] [PubMed]
- Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015;70:323-9. [Crossref] [PubMed]
- Maupeu L, Raguin T, Hengen M, et al. Indications of transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in laryngoscopy, a prospective study of 19 cases. Clin Otolaryngol 2019;44:182-6. [Crossref] [PubMed]
- To K, Harding F, Scott M, et al. The use of Transnasal Humidified Rapid-Insufflation Ventilatory Exchange in 17 cases of subglottic stenosis. Clin Otolaryngol 2017;42:1407-10. [Crossref] [PubMed]
- Gustafsson IM, Lodenius Å, Tunelli J, et al. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) - a physiological study. Br J Anaesth 2017;118:610-7. [Crossref] [PubMed]
- Lau J, Loizou P, Riffat F, et al. The anaesthetic setup prior to and during the implementation of THRIVE in otolaryngological surgery. Asvide 2019;6:218. Available online: http://www.asvide.com/watch/32903
- Lau J, Loizou P, Riffat F, et al. The use of THRIVE in laser surgery. Asvide 2019;6:219. Available online: http://www.asvide.com/watch/32904
- Onwochei D, El-Boghdadly K, Oakley R, et al. Intra-oral ignition of monopolar diathermy during transnasal humidified rapid-insufflation ventilatory exchange (THRIVE). Anaesthesia 2017;72:781-3. [Crossref] [PubMed]
- Humphreys S, Lee-Archer P, Reyne G, et al. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth 2017;118:232-8. [Crossref] [PubMed]
- Riva T, Pedersen TH, Seiler S, et al. Transnasal humidified rapid insufflation ventilatory exchange for oxygenation of children during apnoea: a prospective randomised controlled trial. Br J Anaesth 2018;120:592-9. [Crossref] [PubMed]
Cite this article as: Lau J, Loizou P, Riffat F, Stokan M, Palme CE. The use of THRIVE in otolaryngology: our experiences in two Australian tertiary facilities. Aust J Otolaryngol 2019;2:22.


