
The burden of chronic upper airway disorders in Australia: a population-based cross-sectional study
Introduction
Population-based epidemiologic research facilitates insight into the overall health of citizens, use of healthcare services, which may assist in the planning and development of healthcare policies (1,2). Health surveys distributed to a representative cohort of the population, based on probability sampling, are a useful tool to evaluate disease prevalence and health determinants (3). Specific health determinants of interest may include socio-demographic and economic characteristics, geographical distributions, health and lifestyle behaviours, access to and use of healthcare services, functional capacity, and nutritional status (4). These measures are advantageous to epidemiological research as it facilitates evaluation of numerous health issues and characteristics in both cross-sectional or longitudinal study designs (2).
Chronic upper airway disorders among adults, including allergic rhinitis (AR) and chronic rhinosinusitis (CRS), have been evaluated using population-based health surveys in Europe (5-7), United States (8-11), Canada (12,13), Korea (14), China (15) and Brazil (16). From these studies, the prevalence of AR ranges 6% to 34% and CRS from 5% to 20%. AR and CRS are highly prevalent chronic diseases that contribute to more frequent use of healthcare services, and are associated with greater co-morbidities and reduced productivity (17-19). Prevalence estimates of chronic upper airway disorders vary by sampling method, criteria and country (20-23).
The prevalence of chronic upper airway disorders in Australia has been established in clinical settings, where significant associations have been observed with morbidity and impaired quality of life (24,25). These studies are limited in their generalisability due to sample size restrictions and do not identify socio-demographic, economic and geographic associations that affect the population. A population-based health survey, as used by other countries around the world, can be useful to estimate disease prevalence, identify health determinants and determine the societal impact of chronic upper airway disorders. The National Health Survey (NHS) is administered to a nationally representative sample of Australians and collects information related to socio-demographics, long-term health conditions and lifestyle factors (26). This study’s objective was to determine the epidemiology of AR and CRS in Australia and identify the health determinants that are associated with these conditions.
Methods
This cross-sectional study involved a secondary analysis of data collected from the NHS 2017/18 conducted by the Australian Bureau of Statistics (ABS). Detailed information of the sampling method and other aspects of NHS 2017/18 are published elsewhere (26). Briefly, the sampling method utilized a stratified, multistage cluster sample of households in all states and territories across urban, rural and remote areas, covering 97% of Australia. Respondents included in this survey provided a nationally representative sample of the Australian population. Those excluded from the survey consisted of very remote areas of Australia and discrete Aboriginal and Torres Strait Islander communities, non-private dwellings (hotels, hostels, boarding schools/homes, prisons), overseas visitors residing in Australia for less than 12 months, and households with residents less than 18 years of age. The valid response rate for the NHS 2017/18 was 76%.
Definition of variables
As described by the ABS (26), trained interviewers conducted face-to-face interviews with respondents, focusing on socio-demographic and economic characteristics, lifestyle factors, health status, long-term health conditions, health services use, and medications. Long-term health conditions were coded into a list of 1,000 specific conditions and categories. The list of conditions was developed by the Family Medicine Research Centre at the University of Sydney in consultation with the ABS and guided by the 10th Revisions of the International Classification of Disease (27).
Based on the predetermined questions, individuals participating in this study were asked whether they had any long-term conditions that had lasted or were expected to last for 6 months or more via a prompt card. On this card, ‘hay fever/allergic rhinitis’ and ‘sinusitis or sinus allergy’ were included as discrete options. Given the focus of NHS 2017/18 on long-term health conditions, ‘hay fever/allergic rhinitis’ was considered AR and ‘sinusitis or sinus allergy’ was considered CRS, for the purposes of the current study. Methods used in the current study are similar to those from previously published studies using National Health Surveys in other countries (8-13).
Basic demographics
Individuals less than 15 years of age were excluded from the analytic sample. For demographic characteristics, age was classified using the categories 15–29, 30–44, 45–60, ≥60 years and gender was classified dichotomously (male, female).
Education, employment, and income
Education was considered as the highest level of school completed and defined: tertiary degree, advanced diploma and certificates, high school year 10 to 12, and year 9 and below. Employment consisted of respondents currently employed in full- or part-time occupations and those unemployed or not in the work force.
Income was defined as equivalised weekly personal income ($AUD). Quintiles were constructed by the ABS by ranking personal income in ascending order and dividing the population into five equal groups, each representing 20% of the estimated population.
Cigarette smoking and alcohol consumption
Cigarette smoking status was categorized as an individual-level variable comprised of current, former and never smokers. Current smokers were defined as respondents who smoked daily, weekly or less than weekly. Duration of daily smoking for former or current smokers was categorised as <10, 10 to 19, or ≥20 years of daily smoking. Alcohol consumption was quantified using categories established by the ABS from the National Health and Medical Research Council (NHMRC) for Australia Guidelines for Consumption of Alcohol [2009] (28). Long-term alcohol consumption risk was classified into: exceeding guideline recommendations (average of >2 standard drinks per day), within guideline recommendations (average of ≤2 standard drinks per day) and never consumed alcohol (28).
Statistical analysis
A cross-sectional sample of respondents reporting the presence or absence of AR or CRS were included in the analytic cohort. Sample weights provided by the ABS for the NHS 2017/18 were applied. Odds ratios (OR) and corresponding 95% confidence intervals (95% CI) were calculated to quantify the association between risk factors and AR or CRS. The Chi-squared test was used to assess statistical significance and probability values less than 0.05 were considered statistically significant. A multivariable logistic regression model was constructed to describe the relationship between lifestyle characteristics and likelihood of AR or CRS, after controlling for socio-demographic, educational and economic factors. Unadjusted and adjusted OR were reported for the univariate and multivariate analysis, respectively. Statistical analysis was completed using STATA (StataCorp LP, Stata/IC 14.1, USA, 2016).
Results
The analytical survey population comprised of 17,248 respondents, representing an equivalent of 19,501,433 Australians, aged 45.4±19.1 years, 50.1% females. Most respondents were born in Australia (67.5%), achieved higher than year 12 or equivalent education (87.6%), and were employed (64.3%). Most respondents consumed on average ≤2 standard drinks per day (69.3%) and most never smoked (56.5%). The prevalence of AR was 21.5% and the prevalence of CRS was 9.8%, (representing 4,202,852 and 1,914,494 Australians respectively).
AR
Univariate analysis
AR was significantly more likely in respondents ≤60 than >60 years of age [OR 1.2 (1.1–1.4), P<0.001, Figure 1, Table 1]. The likelihood of AR did not differ between males and females [OR 1.0 (0.9–1.2), P=0.068]. AR was significantly more common among individuals with tertiary education than high school year 9 and below [OR 1.3 (1.1–1.5), P=0.001]. AR was significantly more likely among individuals with part- or full-time employment than those unemployed [OR 1.2 (1.1–1.3), P<0.001]. AR was significantly more common in respondents with personal income ≥$2,610/week than ≤$785/week [OR 1.2 (1.0–1.4), P=0.002].
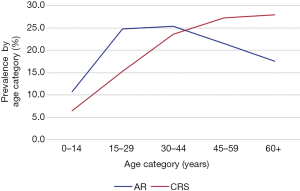
Table 1
| Characteristics | Allergic rhinitis | Chronic rhinosinusitis | |||
|---|---|---|---|---|---|
| OR | P value | OR | P value | ||
| Age group (years) | |||||
| 60+ | 0.8 | 0.016 | 2.3 | <0.001 | |
| 45–59 | 1.0 | 0.558 | 2.1 | <0.001 | |
| 30–44 | 1.1 | 0.054 | 1.7 | <0.001 | |
| 15–29 | Reference | Reference | |||
| Gender | |||||
| Females | 1.0 | 0.068 | 1.5 | <0.001 | |
| Males | Reference | Reference | |||
| Highest level of education | |||||
| Tertiary degree/certificate | 1.3 | 0.001 | 0.7 | 0.009 | |
| Advanced diploma and certificates | 1.2 | 0.023 | 0.8 | 0.268 | |
| High school grades 10 to 12 | 1.0 | 0.288 | 0.6 | <0.001 | |
| Year 9 and below | Reference | Reference | |||
| Employment | |||||
| Unemployed | 0.8 | <0.001 | 1.2 | 0.002 | |
| Full or part-time | Reference | Reference | |||
| Equivalised personal income ($AUD) | |||||
| ≥$2,610/week | 1.2 | 0.002 | 1.0 | 0.783 | |
| $1,726 to 2,610/week | 1.1 | 0.019 | 1.1 | 0.146 | |
| $1,231 to 1,725/week | 1.0 | 0.662 | 1.2 | 0.040 | |
| $786 to 1,230/week | 1.0 | 0.702 | 1.4 | <0.001 | |
| ≤$785/week | Reference | Reference | |||
| Smoking status | |||||
| Current smoker | 0.8 | 0.108 | 1.1 | 0.218 | |
| Former smoker | 1.0 | 0.332 | 1.3 | <0.001 | |
| Never smoked | Reference | Reference | |||
| Lifetime alcohol consumption (2009 NHMRC guidelines) | |||||
| Exceed guidelines: average >2 standard drinks/day | 1.4 | <0.001 | 1.2 | 0.078 | |
| Within guidelines: average of ≤2 standard drinks/day | 1.3 | <0.001 | 1.5 | <0.001 | |
| Never consumed alcohol | Reference | Reference | |||
$AUD, Australian dollars; NHMRC, National Health and Medical Research Council.
AR was significantly more likely in respondents who consumed alcohol than those who did not [>2 standard drinks per day: OR 1.4 (1.2–1.7), P<0.001; ≤2 standard drinks per day: OR 1.3 (1.2–1.6), P<0.001]. In univariate analysis, AR was not associated with smoking status (Table 1).
Multivariate analysis
After controlling for age, gender, education, employment, and income, AR was more significantly more common among individuals ≤60 years of age [adjusted OR: 1.2 (1.1–1.4), P=0.001], females [adjusted OR: 1.1 (1.0–1.2), P=0.010] and individuals who consumed alcohol [adjusted OR 1.4 (1.2–1.6), P<0.001; Figure 2, Table 2].
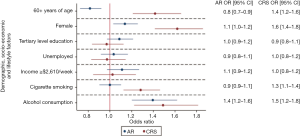
Table 2
| Variables | Allergic rhinitis | Chronic rhinosinusitis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| 60+ years of age1 | 0.8 | 0.7–0.9 | 0.001 | 1.4 | 1.2–1.6 | <0.001 | |
| Female2 | 1.1 | 1.0–1.2 | 0.010 | 1.6 | 1.4–1.8 | <0.001 | |
| Tertiary degree obtained3 | 1.0 | 0.9–1.2 | 0.137 | 0.9 | 0.8–1.1 | 0.683 | |
| Unemployed or not in the labour force4 | 0.9 | 0.8–1.1 | 0.589 | 1.0 | 0.8–1.2 | 0.748 | |
| Personal income ≥$2,610/week5 | 1.1 | 0.9–1.2 | 0.121 | 1.0 | 0.8–1.2 | 0.810 | |
| Cigarette smoking6 | 0.9 | 0.9–1.1 | 0.981 | 1.3 | 1.1–1.4 | <0.001 | |
| Alcohol consumption7 | 1.4 | 1.2–1.6 | <0.001 | 1.5 | 1.2–1.8 | <0.001 | |
Reference groups: 1, <60 years of age; 2, males; 3, highest level of education obtained less than tertiary degree; 4, full- or part-time employment; 5, personal income <$2,610/week; 6, non-cigarette smokers; 7, non-alcohol drinkers. 95% CI, 95% confidence interval.
AR was significantly more likely in respondents consuming ≤2 or >2 standard drinks per day despite adjusting for age, gender, education, employment, income and smoking status [>2 standard drinks per day: adjusted OR 1.5 (1.2–1.8), P<0.001; ≤2 standard drinks per day: adjusted OR 1.4 (1.1–1.6), P<0.001, Figure 3A, Table 3]. Smoking was not associated with AR after adjustment in multivariate models (Table 3, Figure 3).
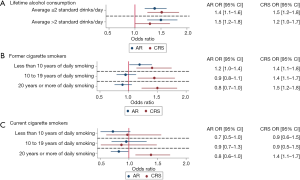
Table 3
| Variables | Allergic rhinitis | Chronic rhinosinusitis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Cigarette smoking | |||||||
| Former1 | |||||||
| 20+ years of daily smoking | 0.8 | 0.7–1.0 | 0.218 | 1.5 | 1.2–1.8 | <0.001 | |
| 10 to 19 years of daily smoking | 0.9 | 0.8–1.1 | 0.636 | 1.4 | 1.1–1.7 | 0.001 | |
| <10 years of daily smoking | 1.2 | 1.0–1.4 | 0.033 | 1.4 | 1.1–1.8 | 0.002 | |
| Current1 | |||||||
| 20+ years of daily smoking | 0.8 | 0.6–1.0 | 0.059 | 1.4 | 1.1–1.7 | 0.003 | |
| 10 to 19 years of daily smoking | 0.9 | 0.7–1.3 | 0.793 | 0.9 | 0.5–1.5 | 0.610 | |
| <10 years of daily smoking | 0.7 | 0.5–1.0 | 0.074 | 0.9 | 0.6–1.5 | 0.907 | |
| Lifetime alcohol consumption (2009 NHMRC guidelines) | |||||||
| Exceed guidelines: average >2 standard drinks/day2 | 1.5 | 1.2–1.8 | <0.001 | 1.2 | 1.0–1.7 | 0.054 | |
| Within guidelines: average ≤2 standard drinks/day2 | 1.4 | 1.1–1.6 | <0.001 | 1.5 | 1.2–1.8 | <0.001 | |
1, adjusted for age, gender, highest level of education, employment, personal income, lifetime alcohol consumption. Reference group: never smokers, <60 years of age, male, year 9 and below high school education, full or part-time employment, personal income ≥$2,610/week, never consume alcohol. 2, adjusted for age, gender, highest level of education, employment, personal income, smoking status. Reference group: never consume alcohol, <60 years of age, male, year 9 and below high school education, full or part-time employment, personal income ≥$2,610/week, never smoke cigarettes. NHMRC, National Health and Medical Research Council.
CRS
Univariate analysis
CRS was significantly more common in respondents >60 than ≤60 years of age [OR 2.3 (1.9–2.9), P<0.001, Figure 1, Table 1]. CRS was significantly more likely among females than males [OR 1.5 (1.3–1.7), P<0.001]. CRS was significantly more common in unemployed respondents than counterparts with part- or full-time employment [OR 1.2 (1.0–1.3), P=0.002].
CRS was significantly more likely in respondents who consumed ≤2 standard drinks per day than those who did not drink alcohol [OR 1.5 (1.2–1.8), P<0.001]. Former smokers were significantly more likely to report CRS than never smokers [OR 1.3 (1.2–1.5), P<0.001]. In univariate analysis, CRS was not significantly more common in current smokers than never smokers (Table 1).
Multivariate analysis
After controlling for age, gender, education, employment, and income, CRS was more significantly more common among individuals >60 years of age [adjusted OR 1.4 (1.2–1.6), P<0.001], females [adjusted OR: 1.6 (1.4–1.8), P<0.001], cigarette smokers [adjusted OR 1.3 (1.1–1.4), P<0.001] and individuals who consumed alcohol [adjusted OR 1.5 (1.2–1.8), P<0.001; Figure 2, Table 2].
CRS was significantly more likely among former smokers who smoked daily for <10 years [adjusted OR 1.4 (1.1–1.8), P=0.002], 10 to 19 years [adjusted OR 1.4 (1.1–1.7), P=0.001] or ≥20 years [adjusted OR 1.5 (1.2–1.8), P<0.001] than non-smokers after controlling for age, gender, education, employment, income and alcohol consumption (Figure 3B, Table 3). Current smokers who smoked daily for ≥20 years were significantly more likely to report CRS than non-smokers [adjusted OR 1.4 (1.1–1.7), P=0.003] after controlling for age, gender, education, employment, income and alcohol consumption (Figure 3C, Table 3).
CRS was significantly more likely among respondents consuming on average ≤2 standard drinks per day than non-drinkers [adjusted OR 1.5 (1.2–1.8), P<0.001] after controlling for age, gender, education, employment, income and smoking status (Figure 3A, Table 3).
Discussion
This study demonstrates that the prevalence of self-reported AR and CRS in Australia are high. Compared to other chronic diseases in Australia, AR (21.5%) is more common than back pain (16.3%), arthritis (15.3%), hypertension (11.3%), and asthma (10.8%) (29). Individuals reporting AR are more likely to be younger, females, and drink alcohol. Compared to other chronic diseases in Australia, CRS (9.8%) is more common than anxiety (9.6%), depression (8.9%), diabetes mellitus (5.1%) and ischaemic heart disease (3.3%) (29). After adjustment, individuals with CRS are more likely to be older, female, cigarette smokers, and drink alcohol. Of cigarette smokers, former smokers and current smokers (who have smoked daily for ≥20 years) are most likely to report CRS than non-smokers after controlling for age, gender, education, employment, income, and alcohol consumption. Of alcohol drinkers, individuals who consume on average ≤2 standard drinks per day are most likely to report AR and CRS than non-drinkers after controlling for age, gender, education, employment, income and smoking status. Overall, this study found that the socio-demographic, economic and lifestyle associations of AR and CRS are congruent with the results of the established literature (6,30).
Globally, the prevalence of AR and CRS differs between countries and may in part be due to variability in the wording of survey questions (20,31). For example, National Health Surveys describe AR singularly or as a combination of ‘hay fever’, ‘nasal allergies’ and ‘rhinitis’. However, survey questions pertaining to CRS tend to use the term ‘sinusitis’ but vary on whether a healthcare professional presented the diagnosis, or if the condition was expected to persist for more than 6 to 12 months. As a result, one of the major limitations of population-based epidemiological studies using face-to-face or telephone interviews—this study included—is the reliance on self-reporting of disease status and thus its susceptibility to reporting bias (32).
To mitigate self-reporting bias, other population-based epidemiological studies have incorporated symptom-specific questions based on clinical practice guidelines and objective assessments (e.g., nasal endoscopy) to confirm diagnosis (33,34). However, adding these components is costly and time consuming. Workman et al. have shown that self-reporting CRS status yields reasonable sensitivity, specificity, positive and negative predictive values compared to tertiary rhinologists diagnosis (35). This suggests that self-reporting may be an inexpensive and valuable screening mechanism to evaluate large cohorts.
In the present study, alcohol consumption is associated with AR and CRS in multivariable regression models. AR and CRS are reported more commonly among individuals who consume alcohol within or exceeding recommended NHMRC guidelines. The link between alcohol and chronic upper airway disorders has been previously established (36). Alcohol consumption has been found to be associated with increased risk of developing perennial AR and is more common among individuals with CRS (37). It is hypothesized that alcohol triggers hypersensitivity reactions via sulphites, reduces T-helper cell type 1 immune responses and increases susceptibility to infection, resulting in greater levels of pro-inflammatory cytokines (38).
Population-based studies have also previously identified a positive correlation between alcohol consumption and total serum IgE and risk of IgE sensitivity (39). Alcohol has also been demonstrated to affect mucosa by dehydrating the epithelial layer, increasing permeability to endotoxins and reducing host defence (40). Alternatively, the relationship between alcohol and CRS may be mediated by gastro-oesophageal reflux disease (GORD) and body size (41). Individuals with CRS who report heartburn and regurgitation also report significantly greater use of alcohol and higher body-mass index (42). GORD is adversely affected by alcohol consumption and is more common in obese individuals (43). Reflux of gastric acid may damage the posterior sinonasal mucosa and mediate neuroinflammatory remodelling contributing to greater symptom burden (44-46).
In this study, current smokers are less likely to report AR or CRS whereas former smokers were more likely to report CRS. However, current smokers who have smoked daily for ≥20 years are more likely to report CRS than non-smokers after adjustment. Smoking has not been found to affect the development of AR (47). Despite this, smoking contributes to elevated serum markers of inflammation and reduced levels of peripheral eosinophils (48). In patients with AR, passive smoking exposure reduces the bioavailability of nitric oxide and contributes to oxidative stress but is not associated with increased symptom burden (49). However, a positive association between smoking and CRS has been demonstrated (47). While tobacco smoke increases ciliary beat frequency, it disrupts the airway surface liquid barrier by inhibiting chloride transport and impairing ciliogenesis (48). A postulated explanation may be that lower concentrations of tobacco smoke act to stimulate a compensatory increase in mucociliary clearance, whereas higher tobacco concentrations cause pathologic changes to sinonasal epithelial cells reducing clearance rates (48). Individuals with a history of second hand smoke sensitivity are more likely to suffer from rhinorrhoea, nasal congestion and headache which may increase risk for developing chronic upper airway inflammation and disease (49).
In this study, the inverse relationship of smoking between AR and CRS may also be affected by age. Individuals with AR were younger, may have less total exposure to tobacco smoke, and may have experienced a compensatory increase in ciliary beat frequency to clear sinuses at a greater than normal rate. Conversely, individuals with CRS were older and may have had greater exposure to tobacco smoke, thereby experiencing pathological changes to sinonasal epithelial cells.
A strength of the present study was its large sample size and representation of the Australian population. However, multiple limitations were present as a secondary analysis was performed using national health survey data, such as relying on self-reported AR and CRS status from pre-determined questions administered by trained interviewers. This method of data collection is subject to self-reporting and recall bias and may overestimate the prevalence of chronic upper airway disorders in the population.
Participants indicated whether they were previously diagnosed with AR or CRS and if these conditions were expected to persist for 6 months or more. Questions such as these are common in epidemiological studies using health survey data and have been evaluated for accuracy (35). Furthermore, survey questions did not use specific validated questionnaires in the diagnosis of AR or CRS, as described in clinical practice guidelines. This also meant that the survey did not account for duration of disease, treatments or disease severity. Additionally, the cross-sectional nature of this study was useful to determine associations but not causality.
Despite these limitations, this study provides valuable insight into the burden of chronic upper airway disorders in Australia. These findings can be used as a platform to develop more comprehensive evaluations that incorporate objective clinical assessments to provide more accurate diagnostic and prognostic results. Future studies are warranted to evaluate the clinical significance of the associations identified in this cross-sectional, population-based epidemiological study. Further research may be directed to evaluate the impact of health and lifestyle behaviours on the management of chronic upper airway disorders and can be useful for implementing public health strategies. For example, the strong association between alcohol and chronic upper airway disorders warrants further investigation to delineate the impact on its symptom burden and response to treatment. The educational, economic and occupational characteristics of AR and CRS may be useful to establish a profile of high-risk individuals, to mitigate future impairment and loss of productivity.
Conclusions
AR and CRS are prevalent chronic upper airway disorders that affect many Australians. Individuals with AR tend to be younger, female, and consume alcohol within or exceeding guideline recommendations. Individuals with CRS tend to be older, female, cigarette smokers and consume alcohol within guideline recommendations. Future studies are warranted to determine clinical significance, societal impact, and effects on health service use, productivity and quality of life.
Acknowledgments
We would like to acknowledge the Australian Bureau of Statistics (ABS) for access to confidential unit files pertaining to the National Health Survey 2017/18 data. Although the research and analysis are based on data collected by the ABS, the opinions expressed do not represent the views of the ABS.
Funding: None.
Footnote
Conflicts of Interest: RC serves as an unpaid editorial board member of Australian Journal of Otolaryngology. LK serves as an unpaid editorial board member of Australian Journal of Otolaryngology. RJH serves as Editor-in-Chief of Australian Journal of Otolaryngology. Richard J. Harvey is a consultant with Medtronic, Olympus and NeilMed pharmaceuticals. He has also been on the speakers’ bureau for Seqirus and MEDA pharmaceuticals. Raymond Sacks is a consultant for Medtronic and Olympus and is in the speaker bureau for Meda Pharmaceuticals. Larry Kalish is on the speaker bureau for Meda Pharmaceuticals, Care Pharmaceuticals and Bayer Pharmaceuticals. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval/informed consent is not required, as the Australian Bureau of Statistics collected survey data in accordance with the National Health Research Medical Council for the purposes of public health research. Secondary analysis of survey data is permissible for research purposes as sensitive characteristics have been removed by the ABS. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gordis L. Epidemiology. 4th ed. Saunders Elsevier; 2008.
- Rothman KJ, Greeland S, Lash TL. Modern Epidemiology. Third. Lippincott Williams & Wilkins; 2012.
- Van den Broeck J, Brestoff J. Epidemiology: Principles and Practical Guidelines. 1st ed. Springer Dordrecht Heidelberg; 2013.
- Marmot M. Social determinants of health inequalities. Lancet 2005;365:1099-104. [Crossref] [PubMed]
- Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe--an underestimated disease. A GA2LEN study. Allergy 2011;66:1216-23. [Crossref] [PubMed]
- Olivieri M, Verlato G, Corsico A, et al. Prevalence and features of allergic rhinitis in Italy. Allergy 2002;57:600-6. [Crossref] [PubMed]
- Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 2004;24:758-64. [Crossref] [PubMed]
- Salo PM, Calatroni A, Gergen PJ, et al. Allergy-related outcomes in relation to serum IgE: Results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol 2011;127:1226-35.e7. [Crossref] [PubMed]
- Arbes SJ, Gergen PJ, Elliott L, et al. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol 2005;116:377-83. [Crossref] [PubMed]
- Bhattacharyya N. Functional limitations and workdays lost associated with chronic rhinosinusitis and allergic rhinitis. Am J Rhinol Allergy 2012;26:120-2. [Crossref] [PubMed]
- Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol 2011;120:423-7. [Crossref] [PubMed]
- Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope 2003;113:1199-205. [Crossref] [PubMed]
- Habib A-RR, Javer AR, Buxton JA. A population-based study investigating chronic rhinosinusitis and the incidence of asthma. Laryngoscope 2016;126:1296-302. [Crossref] [PubMed]
- Kim YS, Kim NH, Seong SY, et al. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy 2011;25:117-21. [Crossref] [PubMed]
- Shi JB, Fu QL, Zhang H, et al. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy 2015;70:533-9. [Crossref] [PubMed]
- Pilan RR, Pinna FR, Bezerra TF, et al. Prevalence of Chronic Rhinosinusitis in Sao Paulo. Rhinology 2012;50:129-38. [Crossref] [PubMed]
- Macdonald KI, McNally JD, Massoud E. The health and resource utilization of Canadians with chronic rhinosinusitis. Laryngoscope 2009;119:184-9. [Crossref] [PubMed]
- Chung SD, Chen PY, Lin HC, et al. Comorbidity profile of chronic rhinosinusitis: A population-based study. Laryngoscope 2014;124:1536-41. [Crossref] [PubMed]
- Liu T, Cooper T, Earnshaw J, et al. Disease burden and productivity cost of chronic rhinosinusitis patients referred to a tertiary centre in Australia. Aust J Otolaryngol 2018;1:5. [Crossref]
- Mims JW. Epidemiology of allergic rhinitis. Int Forum Allergy Rhinol 2014;4:S18-20. [Crossref] [PubMed]
- Katelaris CH, Lee BW, Potter PC, et al. Prevalence and diversity of allergic rhinitis in regions of the world beyond Europe and North America. Clin Exp Allergy 2012;42:186-207. [Crossref] [PubMed]
- Beule A. Epidemiology of chronic rhinosinusitis, selected risk factors, comorbidities, and economic burden. GMS Curr Top Otorhinolaryngol Head Neck Surg 2015;14:Doc11. [PubMed]
- Macdonald KI, Kilty SJ, Van Walraven C. Chronic rhinosinusitis identification in administrative databases and health surveys: A systematic review. Laryngoscope 2016;126:1303-10. [Crossref] [PubMed]
- Hopper JL, Jenkins MA, Carlin JB, et al. Increase in the Self-Reported Prevalence of Asthma and Hay Fever in Adults over the Last Generation: a Matched Parent-Offspring Study. Aust J Public Health 1995;19:120-4. [Crossref] [PubMed]
- Katelaris CH, Sacks R, Theron PN. Allergic Rhinoconjunctivitis in the Australian Population: Burden of Disease and Attitudes to Intranasal Corticosteroid Treatment. Am J Rhinol Allergy 2013;27:506-9. [Crossref] [PubMed]
- Australian Bureau of Statistics. National Health Survey: Users’ Guide, 2017-18 2018. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by Subject/4363.0~2017-18~Main Features~Users’ Guide~1
- World Health Organization. ICD-10: international statistical classification of diseases and related health problems: Tenth revision, 2nd edition. Geneva: 2004.
- National Health and Medical Research Council. Australian Guidelines to Reduce Health Risks from Drinking Alcohol. Canberra, Australia: Commonwealth of Australia; 2009. doi:
10.1037/e509232012-001 . - Australian Bureau of Statistics. National Health Survey: First Results, 2017-18 2018. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by Subject/4364.0.55.001~2017-18~Main Features~Key Findings~1
- Hoffmans R, Wagemakers A, Van Drunen C, et al. Acute and chronic rhinosinusitis and allergic rhinitis in relation to comorbidity, ethnicity and environment. PLoS One 2018;13:e0192330. [Crossref] [PubMed]
- DeConde AS, Soler ZM. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy 2016;30:134-9. [Crossref] [PubMed]
- Novis SJ, Akkina SR, Lynn S, et al. A Diagnostic Dilemma: Chronic Sinusitis Diagnosed by Non- Otolaryngologists. Int Forum Allergy Rhinol 2016;6:486-90. [Crossref] [PubMed]
- Kim JH, Cho C, Lee EJ, et al. Prevalence and risk factors of chronic rhinosinusitis in South Korea according to diagnostic criteria. Rhinology 2016;54:329-35. [Crossref] [PubMed]
- Ahn JC, Kim JW, Lee CH, et al. Prevalence and Risk Factors of Chronic Rhinosinusitus, Allergic Rhinitis, and Nasal Septal Deviation. JAMA Otolaryngol Head Neck Surg 2016;142:162. [Crossref] [PubMed]
- Workman AD, Parasher AK, Blasetti MT, et al. Accuracy of Self-reported Diagnosis of Chronic Rhinosinusitis. Otolaryngol Head Neck Surg 2019;160:556-8. [Crossref] [PubMed]
- Vally H. Allergic and asthmatic reactions to alcoholic drinks: a significant problem in the community. Clin Exp Allergy 2008;38:1-3. [PubMed]
- Bendtsen P, Grnbk M, Kjr SK, et al. Alcohol consumption and the risk of self-reported perennial and seasonal allergic rhinitis in young adult women in a population-based cohort study. Clin Exp Allergy 2008;38:1179-85. [Crossref] [PubMed]
- Friedrich N, Husemoen LLN, Petersmann A, et al. The Association Between Alcohol Consumption and Biomarkers of Alcohol Exposure With Total Serum Immunoglobulin E Levels. Alcohol Clin Exp Res 2008;32:983-90. [Crossref] [PubMed]
- Linneberg A, Berg ND, Gonzalez-Quintela A, et al. Prevalence of self-reported hypersensitivity symptoms following intake of alcoholic drinks. Clin Exp Allergy 2008;38:145-51. [PubMed]
- Thyssen JP, Nielsen NH, Linneberg A. The association between alcohol consumption and contact sensitization in Danish adults: the Glostrup Allergy Study. Br J Dermatol 2008;158:306-12. [Crossref] [PubMed]
- Sella GCP, Tamashiro E, Anselmo-Lima WT, et al. Relation between chronic rhinosinusitis and gastroesophageal reflux in adults: systematic review. Braz J Otorhinolaryngol 2017;83:356-63. [Crossref] [PubMed]
- Choi CW, Kim GH, Soo SC, et al. Is obesity associated with gastropharyngeal reflux disease? Cheol. World J Gastroenterol 2008;14:265-71. [Crossref] [PubMed]
- Lin YH, Chang TS, Yao YC, et al. Increased Risk of Chronic Sinusitis in Adults With Gastroesophgeal Reflux Disease. Medicine (Baltimore) 2015;94:e1642. [Crossref] [PubMed]
- Hanna BC, Wormald PJ. Gastroesophageal reflux and chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg 2012;20:15-8. [Crossref] [PubMed]
- Katle EJ, Hatlebakk JG, Steinsvåg S. Gastroesophageal reflux and rhinosinusitis. Curr Allergy Asthma Rep 2013;13:218-23. [Crossref] [PubMed]
- Katle EJ, Hatlebakk JG, Grimstad T, et al. Gastro-oesophageal reflux in patients with chronic rhinosinusitis investigated with multichannel impedance - pH monitoring. Rhinology 2017;55:27-33. [Crossref] [PubMed]
- Eriksson J, Ekerljung L, Sundblad BM, et al. Cigarette smoking is associated with high prevalence of chronic rhinitis and low prevalence of allergic rhinitis in men. Allergy 2013;68:347-54. [Crossref] [PubMed]
- Reh DD, Higgins TS, Smith TL. Impact of tobacco smoke on chronic rhinosinusitis: a review of the literature. Int Forum Allergy Rhinol 2012;2:362-9. [Crossref] [PubMed]
- Loffredo L, Zicari AM, Occasi F, et al. Passive Smoking Exacerbates Nicotinamide-Adenine Dinucleotide Phosphate Oxidase Isoform 2-Induced Oxidative Stress and Arterial Dysfunction in Children with Persistent Allergic Rhinitis. J Pediatr 2018;202:252-7. [Crossref] [PubMed]
Cite this article as: Habib AR, Campbell R, Kalish L, Wong EH, Grayson J, Alvarado R, Sacks R, Harvey RJ. The burden of chronic upper airway disorders in Australia: a population-based cross-sectional study. Aust J Otolaryngol 2019;2:28.


