
Predicting respiratory complications in paediatric adenotonsillectomy: a risk stratification protocol
Introduction
Sleep disordered breathing (SDB) encompasses a wide range of respiratory conditions ranging from primary snoring to obstructive sleep apnoea (OSA). OSA is the most common form of SDB, affecting 1–2% of normal children (1), and is the most common indication for adenotonsillectomy (AT) in children (2). OSA is characterised by apnoeas, defined in children as prolonged partial upper airway obstruction and/or intermittent complete obstruction, with associated sleep disturbance and/or gas exchange abnormalities (1,3,4). Morbidity related to untreated OSA may manifest as sleep disruption, failure to thrive, cor pulmonale and in severe instances, death. Further neuro-psychological and cognitive consequences can include decreased attention span, lower academic performance and higher rates of behavioural problems (1,5,6). Most otherwise normal children with OSA have adenotonsillar enlargement, and several studies have shown AT is an effective treatment for OSA in children (7,8).
Despite AT relieving mechanical upper airway obstruction in the long-term, children are at higher risk of respiratory compromise in the immediate 24-hour post-operative window, and are traditionally observed overnight after surgery. This risk is 1% in the general paediatric post-AT population, rising to 10–20% in those patients with a diagnosis of OSA (9-11), and has been found to be directly related to the severity of OSA (7,12). This increased risk is thought to have a multi-factorial aetiology including physical factors, such as increased upper airway collapsibility and restriction (13,14), heightened susceptibility to the respiratory depressant effects of anaesthesia and opiates (15,16), and a relatively small airway diameter in children under the age of 2 (15,17,18).
While SDB is a clinical diagnosis, it is hard to determine the severity of OSA based on clinical assessment alone. Polysomnography (PSG) is the gold standard in the diagnosis and categorisation of OSA, but is expensive, not readily accessible and may delay time to surgery. Overnight pulse oximetry has proven to be a useful and more readily available screening test for OSA, with standardised severity scoring methods such as the McGill Oximetry Score developed and validated to a 97% positive predictive value for OSA (7,19). Currently there are few published methods to determine post-operative risk of respiratory adverse events based on pre-operative factors, or an effective risk stratification guideline.
This article is presented in accordance with the STROBE reporting checklist (available http://dx.doi.org/10.21037/ajo-19-75). It aims to evaluate the guideline by comparing the rate of respiratory complications occurring in the 24-hour post-operative period at two hospital sites, and the guideline adherence over the study period. The main outcome measured was the minimisation in the proportion of respiratory adverse events occurring in children at a lower acuity hospital compared to those at a higher acuity hospital, indicating the ability of the peri-operative guideline to successfully stratify patients to appropriately supported sites.
Methods
Monash Health, a large multi-centre health network in Australia encompassing multiple hospital sites with varying levels of post-operative paediatric airway support, developed and systematically implemented a peri-operative risk stratification guideline for patients undergoing AT in 2009. It stratified patients into 3 categories of differing risk of post-operative respiratory compromise, and triaged them to an appropriately supported site. In this way, the protocol aimed to provide an appropriate level of respiratory support to each child, prevent unnecessary inter-hospital transfers and ICU admissions, and efficiently utilise tertiary paediatric care resources.
Approval for this study was obtained from Monash Health (NHMRC approval HREC 13388B).
A retrospective analysis of data from hospital records for patients admitted for elective AT between 2011–2017 was performed. Eligibility criteria included children admitted for either elective tonsillectomy or AT for OSA or SDB between 2011–2017. All children eligible for the study were assessed either in the paediatric otolaryngology clinic or by an otolaryngologist affiliated with the hospital, and patients with a significant history of SDB had either pre-operative oximetry, PSG, or both. Exclusion criteria included age <1 or age >13, tonsillectomy for any indication other than SDB (e.g., recurrent tonsillitis), the absence of pre-operative oximetry or PSG, the absence of documentation pertaining to a patient’s clinical progress in hospital, and the performance of concurrent operations other than grommet insertion, nasal cautery, or coblation of inferior turbinates that could lead to a significant increase in the duration of general anaesthesia and peri-operative morbidity.
Pre-operative overnight oximetry grouped patients into 4 categories based on the McGill Oximetry Score (7):
- Normal/inconclusive data (<3 clusters of desaturation and <3 drops in SpO2 <90%);
- Mild OSA (>3 drops in SpO2 <90% but ≤3 drops in SpO2 <85%);
- Moderate OSA (>3 drops in SpO2 <85% but ≤3 drops in SpO2 <80%);
- Severe OSA (>3 drops in SpO2 <80%).
Pre-operative PSG categorised patients into 4 groups as reported by a sleep physician, guided by the paediatric criteria as defined by AASM, with additional standard predictors such as prolonged hypoventilation or hypoventilation during REM sleep, and oxygen nadir:
- No OSA;
- Mild OSA;
- Moderate OSA;
- Severe OSA.
Patients were then triaged into different care settings based on their pre-operative investigation results and the risk stratification guideline (Figure 1).
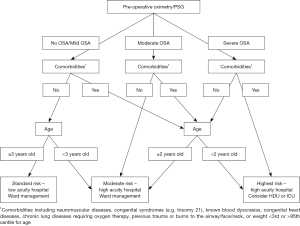
Patients with standard risk of post-operative compromise were managed in a lower acuity hospital with limited after-hours paediatric anaesthetic support. Patients at increased risk underwent surgery at a large quaternary hospital with 24-hour PICU and paediatric anaesthetic support, and were observed on the ward with continuous overnight oximetry. Patients at highest risk were electively managed in PICU with constant monitoring.
All patients received gas induction and intravenous general anaesthesia by a paediatric anaesthetist, as well as weight-appropriate doses of dexamethasone, NSAIDs, short-acting opioid analgesia and ondansetron according to hospital protocol. Tonsillectomy was performed with monopolar cautery and either monopolar or bipolar haemostasis. Adenoidectomy was performed with suction electrocautery. Both adenoidectomy and tonsillectomy were performed using standardised surgical techniques employed at both sites of the health network, and were performed either by consultant surgeons, or registrars under consultant supervision. Simple analgesia was utilised post-operatively, with opioids only used in rare cases for refractory pain.
Patients’ pre-operative factors such as, sex, height, weight and pre-operative respiratory investigation, intra-operative factors such as opioid and dexamethasone doses, and post-operative course in the post-anaesthetic care unit (PACU) and on the surgical ward were followed on hospital records. Respiratory complications were segregated into ‘minor’ complications (desaturations requiring oxygen delivery, apnoeas/obstruction requiring repositioning), and major medical interventions (positive pressure ventilation, intubation or transfer to ICU).
Data were analysed using the SAS software version 9.4 (SAS Institute, Cary, NC, USA). Comparisons between groups (lower versus higher acuity hospital) were made using the Student’s t-test for normally distributed continuous variables, Wilcoxon rank-sum tests for non-normally distributed continuous variables and chi-square or Fisher’s exact test as appropriate for categorical variables. Risk factors for postoperative complications were determined using univariable and multivariable logistic regression analyses. Variables with P<0.05 on univariable analysis or those judged to be clinically important were considered for inclusion in the multivariable regression model. Results from logistic regression analysis were reported as odds ratios (OR) and 95% confidence intervals (95% CI). All calculated P values were two-tailed with P<0.05 indicating statistical significance.
Results
A total of 2,623 patients were considered, and after selection based on the exclusion criteria detailed above, 1,923 patients were enrolled in the study; 904 patients were operated on in the lower acuity centre, and 1,019 at the higher acuity centre (Table 1). There were no statistically significant differences between the groups at both sites with respect to sex, intra-operative opioid and dexamethasone doses, after accounting for weight-related dosage adjustments. Median age and weight centile were higher at the lower acuity hospital; 1,209 patients underwent pre-operative oximetry exclusively, and 688 patients underwent pre-operative PSG; 26 patients had both pre-operative oximetry and PSG, and in these patients, PSG was used preferentially for stratification purposes stratification purposes. One hundred and seventeen patients had major medical comorbidities predisposing to post-operative compromise, 88.9% of which had their operation at the higher acuity centre.
Table 1
| Patient parameter | Lower acuity hospital (n=904) | Higher acuity hospital (n=1,019) | Total (n=1,923) |
|---|---|---|---|
| Male, n (%) | 525 (58.1) | 653 (64.1) | 1,178 (61.25) |
| Age, median year (interquartile range) | 6 [4–7] | 4 [3–5] | 5 [3–6] |
| Age ≤2, n (%) | 12 (1.3) | 213 (20.9) | 225 (11.7) |
| Weight, median kg (interquartile range) | 21 [17–28] | 16 [13–22] | 18.5 [15–25] |
| Weight centile for age, median (interquartile range) | 0.66 [0.47–0.76] | 0.59 [0.35–0.76] | 0.62 [0.43–0.76] |
| Medical comorbidities, n (%) | 13 (1.4) | 104 (10.2) | 117 (6.1) |
| Type of investigation, n (%) | |||
| Oximetry | 691 (76.4) | 518 (50.8) | 1,209 (62.9) |
| PSG | 203 (22.5) | 485 (45.6) | 688 (35.8) |
| Oximetry + PSG | 10 (1.1) | 16 (1.6) | 26 (1.35) |
| Type of operation, n (%) | |||
| Tonsillectomy | 18 (2.0) | 16 (1.6) | 34 (1.8) |
| Adenotonsillectomy | 886 (98.0) | 1,003 (98.4) | 1,889 (98.2) |
| Additional operation, n (%) | 145 (16.0) | 174 (17.1) | 319 (16.6) |
| Dex dose, median mg (interquartile range) | 3 [2–4] | 2.5 [2–3] | 2.5 [2–4] |
| Opioid dose, median morphine mg equivalent (interquartile range) | 5 [3.5–8] | 4.5 [3.5–7] | 5 [3.5–7] |
| Complications | |||
| Intra-operative complication, n (%) | 1 (0.1) | 3 (0.3) | 4 (0.2) |
| Total complication, n (%) | 11 (1.2) | 137 (13.4) | 148 (7.7) |
| Minor respiratory complications, n (%) | 11 (1.2) | 120 (11.8) | 131 (6.8) |
| Airway obstruction requiring repositioning | 4 | 26 | 30 |
| Desaturation requiring prolonged (>1 hour) oxygen | 4 | 65 | 69 |
| Both repositioning and prolonged oxygen | 3 | 37 | 40 |
| Major respiratory complications, n (%) | 0 (0.0) | 16 (1.6) | 16 (0.8) |
| Non-invasive ventilation | 0 | 7 | 7 |
| Transfer to ICU | 0 | 9 | 9 |
| Recovery room complication, n (%) | 4 (0.4) | 9 (0.9) | 13 (0.7) |
PSG, polysomnography.
Overall complication rates at the lower acuity centre were 1.2%, compared with 13.4% at the higher acuity centre. After adjusting for potential confounding variables, patients at the higher acuity centre had 11.68-fold increased odds of post-operative respiratory complication. All 11 complications at the lower acuity hospital were minor complications involving either repositioning or oxygen therapy that did not delay discharge, and patients were all discharged day 1 post-op. There was one intra-operative non-respiratory complication requiring inter-hospital transfer.
The odds of certain pre-operative and peri-operative variables leading to post-operative respiratory compromise were calculated on univariable analysis. Potential confounders were considered and adjusted for on multivariable analysis (Table 2), with analysis A comparing the two care settings, and analysis B comparing independent pre-operative and peri-operative risk factors. The odds of respiratory complications in patients with severe OSA on either oximetry or PSG were 6.38 times higher than patients with no OSA (95% CI, 3.72–10.94; P<0.0001). Table 3 compared oximetry and PSG as pre-operative screening methods. Severe OSA conferred similar increased odds when discovered on oximetry (OR, 8.54; 95% CI, 4.70–15.50; P<0.0001) or PSG (OR, 7.89; 95% CI, 1.05–59.01; P=0.044). Other pre-operative factors contributing to post-operative risk included age ≤2 (OR, 2.65; 95% CI, 1.69–4.15; P<0.0001), congenital syndromes (OR, 2.67; 95% CI, 1.25–5.68; P=0.011), and chronic lung disease (OR, 3.89; 95% CI, 1.06–14.20; P=0.04). Sex, weight ≤5th centile or >95th centile for age, the presence of neuromuscular disorders or cardiac conditions, opiate and dexamethasone doses did not contribute a statistically significant risk. Additional independent factors included the presence of additional procedures such as coblation of inferior turbinates, nasal cautery or insertion of grommets (OR, 1.73; 95% CI, 1.12–2.68, P=0.013), and post-operative recovery room complications (OR, 9.28; 95% CI, 2.41–35.77; P=0.001).
Table 2
| Variable | Odds ratio | Lower limit 95% CI | Upper limit 95% CI | P value |
|---|---|---|---|---|
| Multivariable analysis model A: higher acuity centre vs. lower acuity centre | ||||
| Higher acuity centre | 11.68 | 6.25 | 21.81 | <0.0001 |
| Male | 1.33 | 0.91 | 1.95 | 0.139 |
| Opiate dose | 0.98 | 0.94 | 1.03 | 0.429 |
| Dexamethasone dose | 1.04 | 0.89 | 1.20 | 0.634 |
| Additional operation1 | 2.05 | 1.36 | 3.08 | 0.001 |
| Multivariable analysis model B: independent pre-operative and peri-operative risk factors | ||||
| Recovery room complication | 9.28 | 2.41 | 35.77 | 0.001 |
| Severe OSA2 | 6.38 | 3.72 | 10.94 | <0.0001 |
| Moderate OSA3 | 3.96 | 2.09 | 7.50 | <0.0001 |
| Mild OSA4 | 2.16 | 1.03 | 4.54 | 0.042 |
| Chronic lung disease | 3.89 | 1.06 | 14.20 | 0.040 |
| Congenital syndrome | 2.67 | 1.25 | 5.68 | 0.011 |
| Age ≤2 | 2.65 | 1.69 | 4.15 | <0.0001 |
| Additional operation | 1.73 | 1.12 | 2.68 | 0.013 |
| Male | 1.35 | 0.91 | 2.01 | 0.133 |
| ≤5th weight centile for age | 0.73 | 0.32 | 1.66 | 0.460 |
| ≥95th weight centile for age | 1.44 | 0.84 | 2.45 | 0.187 |
| Neuromuscular disorder | 1.61 | 0.40 | 6.53 | 0.507 |
| Congenital heart disease | 1.03 | 0.32 | 3.30 | 0.959 |
| Opiate dose | 0.99 | 0.94 | 1.04 | 0.660 |
| Dexamethasone dose | 1.00 | 0.85 | 1.16 | 0.961 |
| Intra-operative complication | 7.37 | 0.93 | 58.27 | 0.058 |
1, the presence of additional operations including coblation of inferior turbinates, nasal cautery and insertion of grommets; 2, diagnosis of severe OSA with either an oximetry or PSG score of 4; 3, diagnosis of moderate OSA with either an oximetry or PSG score of 3; 4, diagnosis of mild OSA with either an oximetry or PSG score of 2. OSA, obstructive sleep apnoea; PSG, polysomnography.
Table 3
| Variable | Odds ratio | Lower limit 95% CI | Upper limit 95% CI | P value |
|---|---|---|---|---|
| Subgroup analysis A: oximetry | ||||
| Male | 1.20 | 0.71 | 2.04 | 0.489 |
| Age ≤2 | 2.90 | 1.60 | 5.25 | 0.0004 |
| Mild OSA | 2.03 | 0.76 | 5.44 | 0.157 |
| Moderate OSA | 3.73 | 1.70 | 8.18 | 0.001 |
| Severe OSA | 8.54 | 4.70 | 15.50 | <0.0001 |
| Congenital disorder | 4.09 | 0.99 | 16.87 | 0.051 |
| Opioid dose | 1.00 | 0.94 | 1.06 | 0.948 |
| Dexamethasone dose | 0.98 | 0.78 | 1.24 | 0.882 |
| Additional operation | 2.38 | 1.36 | 4.19 | 0.003 |
| Recovery room complication | 22.43 | 3.55 | 141.85 | 0.001 |
| Subgroup analysis B: PSG | ||||
| Male | 1.63 | 0.90 | 2.94 | 0.104 |
| Age ≤2 | 3.23 | 1.66 | 6.28 | 0.001 |
| Mild OSA | 3.28 | 0.39 | 27.54 | 0.274 |
| Moderate OSA | 6.11 | 0.77 | 48.31 | 0.086 |
| Severe OSA | 7.89 | 1.05 | 59.01 | 0.044 |
| Congenital disorder | 2.84 | 1.23 | 6.53 | 0.014 |
| Opioid dose | 0.99 | 0.92 | 1.06 | 0.693 |
| Dexamethasone dose | 1.10 | 0.91 | 1.34 | 0.882 |
| Additional operation | 1.04 | 0.52 | 2.10 | 0.904 |
| Recovery room complication | 2.84 | 0.27 | 30.06 | 0.386 |
OSA, obstructive sleep apnoea; PSG, polysomnography.
Of the 700 patients that were excluded from the study, 674 were excluded due to the lack of either pre-operative oximetry or PSG indicated by the protocol. Accounting for the 26 patients excluded for other reasons, adherence to the protocol with respect to pre-operative investigation of OSA over the study period was 74.3%. Agreement between the hospital site predicted by the protocol, and the actual site that patients were admitted to for their surgery over the study period was 97%.
Discussion
The results of this study show that the stratification protocol implemented at Monash Health, Australia, is highly effective in identifying children at risk of post-operative respiratory compromise when undergoing AT and placing them in an appropriate care setting. While, as expected, the protocol did not decrease the absolute risk of post-operative respiratory complications, with the total complication rate of 7.7% across all sites consistent with previous studies, it ensured higher risk patients were cared for in a care setting equipped to manage potential complications. The successful implementation of this protocol has contributed to 11.55-fold higher odds of respiratory complications occurring in a hospital able to appropriately manage such complications. In this study, there was only 1 inter-hospital transfer, a 3-year-old female who developed new onset atrial tachycardia intra-operatively that could not have been predicted on pre-operative investigation and triage, requiring inter-hospital transfer for further observation in ICU. In the 7 years assessed in this study, there were no major respiratory complications at the lower acuity centre.
After adjusting for potential confounders, it was found that the strongest contributor to peri-operative risk was severity of OSA, with severe OSA imparting 6.38-fold increased odds of post-operative respiratory compromise, consistent with other published studies (7,12,18,20,21). Wilson et al. found that children with a pre-operative overnight saturation nadir ≤80% or apnea-hypopnea index (AHI) ≥5 had likelihood ratios for post-operative respiratory compromise of 3.1 and 1.7 respectively (17). Sanders et al. found children with an RDI 30 or greater were more likely to experience laryngospasm on emergence and oxygen desaturation <92% on emergence (12). In this study, children aged ≤2 had 2.65-fold increased odds for respiratory compromise, and is consistent with findings in the literature (11,21,22). A large (n>2,000) study found children younger than 3 years had a nearly 2-fold increased risk of post-operative respiratory complications (21), and another study found children under 2-years-old had an adjusted OR of 4.3 for respiratory compromise (95% CI, 1.7–11) (17). Weight is another documented risk factor in the literature. One study found the odds of PICU admission in obese patients was 10.6 times higher (P=0.00001) than non-obese patients (23), and another found patients with respiratory complications were more likely to weigh less than the 5th percentile for age (OR 5.1, 95% CI, 1.4–18.7) (11). However, in this study, there was no statistically significant increased odds of respiratory compromise in weight ≤5th or ≥95th centile for age. Patients’ progress within the recovery room was also highly predictive of further respiratory complications on the ward, with respiratory room complications conferring 9.28-fold increased odds of respiratory complication on the ward. The negative predictive value of a complication free recovery room period was 92.34% (95% CI, 92.12–92.56), reflecting previous findings that routine post-operative ICU admission for high risk children may be avoided with close monitoring in the recovery room, with a negative predictive value of an uncomplicated course in PACU of 98.3% (24). This emphasizes the importance of recovery room assessment in the triaging between care settings within a hospital, which may decrease the burden of unnecessary elective ICU admissions, and conversely flag patients initially deemed fit for ward who suffered a recovery room complication to be referred for closer monitoring in ICU.
There has been a growing shift towards day-case tonsillectomies, and the findings of this study may support the case for day procedure tonsillectomies in the right patient subgroup. Results indicate that respiratory complications are rare in patients deemed at low risk of post-op respiratory compromise (1.2%), and a complication-free recovery room progress confers NPV 92.34% for early post-op respiratory compromise. However, there are many other factors in the decision making of keeping a patient for overnight observation, including monitoring for primary post-tonsillectomy bleed, and ensuring adequate analgesia and oral intake prior to discharge. Thus, the findings in this study may further assist clinicians in their decision-making process when triaging children into day-stay vs. overnight stay adenotonsillectomies.
Within subgroup analyses, severe OSA on oximetry had 8.54-fold increased odds of post-operative respiratory compromise, similar to the OR of 7.89 for PSG-diagnosed severe OSA. Along with previous studies showing a strong relation between increasing oximetry scores and the need for post-operative respiratory intervention (7,19), this study further highlights the value in pre-operative overnight oximetry as a cost-efficient and time-effective alternative to PSG for planning pre-operative care.
Protocol adherence rate was 97% between hospital sites. Of the 924 patients triaged to the lower acuity centre based on the protocol, 904 received their operation there. Thirteen patients with medical comorbidities received their operations at the lower acuity centre following triage by a consultant anaesthetist against protocol advice, indicating 88.9% adherence within this subgroup. Only 1 of these patients required prolonged post-operative oxygen delivery. Within care settings, only 33.0% of patients triaged for ICU admission were actually admitted to ICU post-operatively, which could be explained by the aforementioned finding that an uncomplicated PACU admission predicted a complication free admission (24), and thus, children initially destined for ICU had their care de-escalated to ward care following anaesthetist guidance. This may indicate that while the protocol is useful in determining the best care setting between hospitals, there is an importance in clinician assessment in the recovery room setting.
There have been similar protocols documented in the literature. The John Hunter Children’s Hospital (Newcastle, Australia) identified certain criteria for elective admission to PICU post AT: RDI >60, age <3, weight <3rd centile, comorbidities associated with postoperative airway obstruction and a component of central apnoea greater than 30% (20). Walker et al. found that a REM RDI >60 warranted elective same-day PICU admissions, with review by surgeon and intensive care physician prior to discharge to paediatric surgical ward overnight (20). The Nationwide Children’s Hospital in Ohio, USA, use a guideline to triage children for AT either at the children’s hospital or outpatient surgery centres as day cases, based on risk factors in the literature (age <3, sleep study findings, BMI >95% for age, and other relevant comorbidities). This study revealed a decrease in unanticipated admissions from 88 (2.38%) to 43 (1.44%) after implementation of the guideline, where 42 percent of unanticipated admissions prior to the implementation of the guidelines would have met criteria for admission. However, fewer than 10% of patients in the study underwent any preoperative investigation for a formal diagnosis and characterisation of severity of OSA (25).
The strengths of this study lie in its large sample size and statistical significance over a prolonged study duration of 7 years. The high protocol adherence of 97% allowed for an accurate comparison of the complication rates between sites. It is also highly translatable to other care networks around the world, particularly for consideration of safe AT in remote areas that may not have access to PSG, or specialised paediatric anaesthetic and ICU support.
Limitations of this study include its retrospective design and the heterogeneity of the study population. Analysis did not include a formal comparison of complication rates of the two hospitals prior to the introduction of the protocol, and hence it cannot be definitively stated that the discrepancy between the complication rates at the two sites are due to the protocol alone. A preceding unpublished before/after study by one of the senior authors compared the complication rates between sites in the two years prior to the implementation of the protocol, with the two years after protocol introduction. While the study did not reach statistical significance, there was clear evidence of a decrease in the proportion of complications at the peripheral site and a concurrent increase in the complication rates at the central hospital in the 2 years post protocol implementation, indicating an improved ability to stratify patients to appropriate care settings as a result of the protocol.
Our current study leaves questions for potential further research. Given the strong association between complications within the recovery room and on the ward, there may be value in including a recovery room assessment as an additional component of the existing protocol. Further, a cost-effectiveness analysis of the protocol would also be informative. Lastly, follow up studies regarding the generalisability of the protocol to other settings, in particular rural and regional settings performing AT, in order to further minimise risk of compromise in an unsupported setting, would be useful.
Conclusions
This study highlights the importance of diagnosis and quantification of OSA by either oximetry or PSG, and assessment of certain pre-operative factors that successfully predict risk of post-operative respiratory complications in children post AT. Severe OSA, age under 2 years, presence of congenital abnormalities associated with airway compromise, additional procedures increasing anaesthetic duration, and respiratory room complications all were statistically significant risk factors for post-operative respiratory compromise. This protocol and its associated findings have important implications and can guide decision making regarding the most appropriate care setting of patients undergoing AT.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-19-75
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-19-75). PP serves as an unpaid editorial board member of the Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. The study protocol was approved by the Institutional Review Board of Monash Health (NHMRC approval HREC 13388B). Due to the retrospective nature of this study, the need for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nixon GM, Brouillette R. Sleep·8: Paediatric obstructive sleep apnoea. Thorax 2005;60:511-6. [Crossref] [PubMed]
- Bluestone CD. Current indications for tonsillectomy and adenoidectomy. Ann Otol Rhinol Laryngol Suppl 1992;155:58-64. [Crossref] [PubMed]
- Caulfield HM. Obstructive Sleep Apnoea in Childhood. 7 ed. Gleeson M. editor. Oxford: Hodder Arnold; 2008.
- Powell S, Kubba H, O’Brien C, et al. Paediatric obstructive sleep apnoea. BMJ 2010;340:c1918. [Crossref] [PubMed]
- Brunetti L, Rana S, Lospalluti ML, et al. Prevalence of obstructive sleep apnea syndrome in a cohort of 1,207 children of southern Italy. Chest 2001;120:1930-5. [Crossref] [PubMed]
- Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics 1998;102:616-20. [Crossref] [PubMed]
- Nixon GM, Kermack AS, Davis GM, et al. Planning adenotonsillectomy in children with obstructive sleep apnea: the role of overnight oximetry. Pediatrics 2004;113:e19-e25. [Crossref] [PubMed]
- Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr 1982;100:31-40. [Crossref] [PubMed]
- Richmond KH, Wetmore RF, Baranak CC. Postoperative complications following tonsillectomy and adenoidectomy--who is at risk? Int J Pediatr Otorhinolaryngol 1987;13:117-24. [Crossref] [PubMed]
- Rosen GM, Muckle RP, Mahowald MW, et al. Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics 1994;93:784-8. [PubMed]
- McColley SA, April MM, Carroll JL, et al. Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 1992;118:940-3. [Crossref] [PubMed]
- Sanders JC, King MA, Mitchell RB, et al. Perioperative Complications of Adenotonsillectomy in Children with Obstructive Sleep Apnea Syndrome. Anesth Analg 2006;103:1115-21. [Crossref] [PubMed]
- Marcus CL, McColley S, Carroll J, et al. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol 1994;77:918-24. [Crossref] [PubMed]
- Gozal D, Burnside MM. Increased upper airway collapsibility in children with obstructive sleep apnea during wakefulness. Am J Respir Crit Care Med 2004;169:163-7. [Crossref] [PubMed]
- Waters KA, McBrien F, Stewart P, et al. Effects of OSA, inhalational anesthesia, and fentanyl on the airway and ventilation of children. J Appl Physiol 2002;92:1987-94. [Crossref] [PubMed]
- Brown KA, Laferriere A, Lakheeram I, et al. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology 2006;105:665-9. [Crossref] [PubMed]
- Wilson K, Lakheeram I, Morielli A, et al. Can assessment for obstructive sleep apnea help predict post-adenotonsillectomy respiratory complications. Anesthesiology 2002;96:313-22. [Crossref] [PubMed]
- Nixon GM, Kermack AS, McGregor CD, et al. Sleep and breathing on the first night after adenotonsillectomy for obstructive sleep apnea. Pediatr Pulmonol 2005;39:332-8. [Crossref] [PubMed]
- Brouillette RT, Morielli A, Leimanis A, et al. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics 2000;105:405-12. [Crossref] [PubMed]
- Walker P, Whitehead B, Rowley M. Elective admission to PICU after adenotonsillectomy for severe obstructive sleep apnoea. Anaesth Intensive Care 2007;35:453. [PubMed]
- Statham MM, Elluru RG, Buncher R, et al. Adenotonsillectomy for Obstructive Sleep Apnea Syndrome in Young Children: Prevalence of Pulmonary Complications. Arch Otolaryngol Head Neck Surg 2006;132:476-80. [Crossref] [PubMed]
- Thongyam A, Marcus CL, Lockman JL, et al. Predictors of perioperative complications in higher risk children after adenotonsillectomy for obstructive sleep apnea: a prospective study. Otolaryngol Head Neck Surg 2014;151:1046-54. [Crossref] [PubMed]
- Tweedie DJ, Bajaj Y, Ifeacho S, et al. Peri-operative complications after adenotonsillectomy in a UK pediatric tertiary referral centre. Int J Pediatr Otorhinolaryngol 2012;76:809-15. [Crossref] [PubMed]
- Theilhaber M, Arachchi S, Armstrong DS, et al. Routine post-operative intensive care is not necessary for children with obstructive sleep apnea at high risk after adenotonsillectomy. Int J Pediatr Otorhinolaryngol 2014;78:744-7. [Crossref] [PubMed]
- Raman VT, Jatana KR, Elmaraghy CA, et al. Guidelines to decrease unanticipated hospital admission following adenotonsillectomy in the pediatric population. Int J Pediatr Otorhinolaryngol 2014;78:19-22. [Crossref] [PubMed]
Cite this article as: Chia C, Haran S, Wong T, Lam K, Nixon GM, Paul E, Paddle P. Predicting respiratory complications in paediatric adenotonsillectomy: a risk stratification protocol. Aust J Otolaryngol 2020;3:21.


