
Laryngeal chondrosarcoma of the cricoid cartilage—a case series towards conservative management
Introduction
Laryngeal chondrosarcoma (LC) is a rare malignancy accounting for 0.2–2% of laryngeal malignancies (1). They are of mesenchymal origin typically arising in the axial skeleton. Although the pathophysiology is unknown, they are believed to arise from abnormal ossification of hyaline cartilages and rarely elastic cartilages (2). Other theories proposed include persisting cartilaginous remnants, pluripotent stem cells and ischaemic change, although these are less favored (3). To date there are just over 600 cases reported in the literature (4-6).
LCs typically affect people in the 5th to 7th decades of life with an average age of 62.5 years and a male to female ratio of 3:1 (1,4). The most common symptoms include chronic dysphonia, dysphagia and neck mass. Nasoendoscopy demonstrates a smooth mass, which may affect vocal cord movement if the cricoarytenoid joint is involved (Figure 1A). Given the indolent nature of the disease, diagnosis is often delayed.
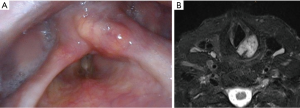
These tumours have a predilection toward affecting the hyaline cartilage of the cricoid followed by the thyroid cartilage and less frequently the epiglottis and arytenoid cartilage (4,7). Computed tomography is usually the initial imaging modality of choice showing a typical “popcorn calcification” appearance either within the confines of the cartilaginous margin or beyond into surrounding tissues (1,8). In the case of invasion into the surrounding tissues, magnetic resonance imaging may be useful for determining extent of soft tissue spread (Figure 1B) (2). More recently a hybrid of positron emission tomography/magnetic resonance imaging (PET/MRI) has been reported especially for more aggressive subtypes (9).
Histological diagnosis is based on the criteria described by Lichtenstein and Jaffe and adapted by the World Health Organisation Classification grading of soft tissue and bone tumours (10). Histologically, LC is differentiated by grade (low, moderate, high) and morphologic subtype (morphoeic) (1). Other rare variations include dedifferentiated, clear cell, myxoid and chondrosarcoma with additional malignant mesenchymal component (4) (Table 1). Although chondrosarcomas of the extremity, trunk, skull and facial bones can be staged according to the AJCC tumour (T), node (N) and metastases (M) system, there is no tumour specific TNM system for LC (11).
Table 1
| Grade/type | Histological description |
|---|---|
| Grade 1 | Moderately cellular |
| Hyperchromatic, plump nuclei of uniform size | |
| Occasional binucleated cells present | |
| Absent mitosis | |
| Grade 2 | More cellular |
| Greater degree of nuclear atypia, hyperchromasia and nuclear size | |
| Mitoses present | |
| Grade 3 | Cellular, pleomorphic and atypical |
| Less differentiation and cell spindling | |
| Dedifferentiated chondrosarcoma | Malignant variant |
| Characterised by bimorphic histological appearance | |
| o Distinct areas of low-grade chondrosarcoma and higher grade non-cartilaginous sarcoma | |
| Mesenchymal chondrosarcoma | Characterised by a bimorphic pattern |
| o Poorly differentiated small round/oval cells with scant cytoplasm and islands of well-differentiated hyaline cartilage | |
| Clear cell chondrosarcoma | Characterized by proliferation of tumour cells with clear cytoplasm |
Treatment options vary according to the location, size of tumour and patient symptoms. This may range from observation to total laryngectomy.
Recently, Chin et al. published a review of 592 cases of LC where interestingly, a total laryngectomy was offered in 29.4% second only to local excision (30.1%) (4). Despite its low metastatic rate, recurrence is high even with aggressive treatment given the locally aggressive nature of the disease (6).
This study provides an Australian experience advocating for conservative approaches to management including monitoring to function preserving operations. Given the slow growing nature of the disease and high recurrence rates despite the treatment offered, a laryngectomy should only be reserved for very select cases. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-19-84).
Methods
This study was approved by the institutional human research ethics committee (Number: 1909-02). A retrospective review of patients was carried out between 2011 and 2018 at the Department of Otolaryngology Head and Neck Surgery at Westmead Hospital.
Patients were included if they had biopsy proven LC. Data on patient symptoms, relevant imaging, operative reports and follow up were recorded.
A literature review was conducted using MEDLINE, EMBASE and PubMed with the following terms: “Layn* OR Cricroid OR Thyroid cartilage OR Epiglottis OR Glottic OR Supraglottic OR Subglottic OR Vocal cords” AND “Chondro*”. This search was limited to humans and adults >18 years of age. Studies that were limited to the English language and published after 1946 were included.
Results
Patient characteristics
A total of six cases were included with a male to female ratio of 5:1, ages ranging from 50–81 years old. The most common symptoms were dysphonia and stridor. Four out of six patients were smokers. One patient was immunocompromised with a long-standing kidney transplant. A summary is provided in Table 2. Computed tomography was the initial imaging of choice for all patients followed by MRI to assess for soft tissue extension and spread. No patient had any evidence of regional lymph node spread or distant metastases.
Table 2
| Patient | Age, year | Gender | Presenting symptoms | Tumour location | Tumour grade | Treatment offered | Follow up |
|---|---|---|---|---|---|---|---|
| 1 | 67 | M | Hoarseness, stridor | Cricoid cartilage with subglottic extension | Grade 1 | Endoscopic debulking | AWD, 6 years |
| 2 | 50 | M | Hoarseness, stridor | Right cricoid cartilage | Grade 1 | Right partial laryngectomy | AWD, 2 years |
| 3 | 55 | M | Hoarseness, stridor | Right cricoid cartilage | Not specified | Endoscopic debulking | AWD, 12 years |
| 4 | 62 | F | Hoarseness | Left cricoid cartilage | Grade 1 | Endoscopic cricoid split—diagnostic | AWD, 2 years |
| 5 | 81 | M | Dyspnoea, stridor | Cricoid cartilage with subglottic extension | Grade 2 | Endoscopic debulking | AWD, 10 years |
| 6 | 56 | M | Dysphonia, globus | Left cricoid cartilage, left cricoarytenoid joint and arytenoid cartilage | Grade 1 | Monitoring | AWD, 2 years |
AWD, alive with disease.
Tumour characteristics
The tumour sites included 2 patients with posterior cricoid cartilage involvement and extension into the 1st tracheal ring, 2 patients with left posterior cricoid and 2 with right cricoid cartilage involvement. All tumours were biopsy proven chondrosarcoma. Each specimen required a secondary review from the pathology lab and diagnosis was reached after a multidisciplinary team meeting. Four patients had grade 1 chondrosarcoma, 1 patient had grade 2 and one patient had chondrosarcoma unspecified being reported as “atypical low grade”.
Treatment and follow up
Five patients underwent local excision and debulking with one patient requiring an endoscopic cricoid split approach. Among these, one patient subsequently underwent a partial laryngectomy including a right hemicricoid and right thyroid lamina resection. The aim of this surgery was debulking rather than total excision given the multiple margins of the specimen that were involved following resection. This was later followed by laser resection and partial arytenoidectomy due to nocturnal airway obstruction and anterior prolapse of the right arytenoid.
Two patients (patient 2 and patient 4) needed a tracheostomy tube during their operation and were subsequently decannulated. All patients were discharged within 5 days of admission. One patient underwent a core biopsy and following confirmation of a low-grade chondrosarcoma received no further treatment.
Median follow time was 48 months. At the time of this writing, all patients were alive with no significant disease progression affecting patient symptomatology. All patients are continuing to be monitored at 6–12 monthly intervals with serial imaging.
Discussion
Although LC is the fifth most common laryngeal malignancy, its incidence is relatively small making diagnosis and management difficult (5).
Current histologic criteria follows that of malignant cartilaginous tumours as described by Lichtenstein and Jaffe (12) and later modified by Evans et al. (13).
Histologically this is characterized by nuclear atypia and hypercellularity (many cells with plump nuclei, multi-nucleate cells or giant cells) (12). The mitotic rate, cellularity and nuclear size further determine grade (13). Grade one chondrosarcomas account for the majority (64%) of cases followed by grade 2 (28%).
Some LCs can erroneously be diagnosed as benign chondromas. To ensure an accurate histological diagnosis, many authors recommend robust pathological sampling as often multiple reviews and multiple opinions may be required.
In our series one patient underwent three unsuccessful biopsies eventually requiring an endoscopic cricoid split approach to obtain a sufficient biopsy. Fine needle aspirations are usually unsuccessful and are usually unable to distinguish between a chondrosarcoma and a benign chondroma (4).
In cases where histological diagnosis is difficult or if the tumour sample is small, electron microscopy has been advocated for examination of the tumour ultrastructure. LC can be characterised by dominant mitochondria, fat vacuoles, dilated rough endoplasmic reticulum and chondrocyte morphology (14).
A systematic review by Chin and colleagues (4) reported 44.6% of patients received either a total or partial laryngectomy. Other surgical modalities favoring more conservative treatment options such as endoscopic or laser excision accounted for approximately 9%. In this series 3 patients underwent local excision to debulk the tumour and 2 patients underwent no treatment after histological diagnosis was confirmed. One patient underwent a partial laryngectomy including resection of right hemi-cricoid and thyroid lamina due to the obstructive nature of the disease.
Various conservative surgical approaches have been described focusing predominantly on laryngeal preservation (5,15-18). This is achieved by way of preserving at least one cricoarytenoid unit as well as providing structural support at the level of the cricotracheal junction (19).
Common surgical options include endoscopic resection, open neck partial laryngectomy and cricotracheal resection with anastomosis.
Endoscopic trans-oral resection has been advocated largely for diagnostic evaluation and for obtaining a biopsy with robust sampling. In select cases, it may be offered as a therapeutic option particularly in cases where LC does not involve the cricoid cartilage (7,16,20). Recently, transoral robotic surgery for resection has also been described allowing for better exposure, lower patient morbidity and comparable outcomes to traditional surgical options (21).
In cases of LC affecting the thyroid cartilage, arytenoids or epiglottis, a supracricoid partial laryngectomy is a feasible option allowing for functional organ preservation (15,16,22). However, LC involving the cricoid cartilage is difficult to manage, with most authors favoring a surgical approach (5).
In a recent review, Piazza and colleagues (19) compared different surgical techniques for LC affecting the cricoid cartilage, proposing resection of the laryngeal framework with a defect-oriented reconstructive approach.
Reconstruction involves either an end-to-end anastomosis or interposition grafts of vascularized tissue depending on the amount of cricoid cartilage involved.
LC affecting the cricoid arch alone may be reconstructed with a thyro-crico-tracheal anastomosis without affecting structural airway stability.
In cases where the defect involves less than 75% of the cricoid plate with preservation of one crico-arytenoid joint, reconstruction is aimed at providing unilateral structural posterior support with various reconstructive options described including a tracheo-hyoido-epiglottopexy, hyoid-sternohyoid pedicled flap, tracheal auto-transplantation, revascularized cartilage grafts or tip of scapula free flap (19,23-25).
In large subtotal or total defects involving both crico-arytenoid joints, the aim of reconstruction would be to provide rigid posterior structural support which may necessitate microvascular anastomosis such as a rotational thyro-tracheal anastomosis or a medial femoral condyle free flap (19,23,25,26).
Although a total laryngectomy was the second most common surgical option offered in a systematic review by Chin et al. (4), the focus has shifted towards more conservative options. There remain few indications for a total laryngectomy including bulky tumours with multiple cartilages involved or for aggressive chondrosarcomas (high grade or de-differentiated) (16). Even in cases of recurrent LC, a total laryngectomy did not confer better outcomes than conservative surgery (2). A neck dissection should also be reserved for exceptional cases where regional lymph node metastasis is present (27).
There is little published data on the role of radiotherapy as a treatment modality. In the systematic review by Chin et al., only 0.8% of patients had radiotherapy alone and even less (0.2%) received chemotherapy as a sole treatment. Adjuvant radiotherapy/chemotherapy was more common. Some authors believe chondrosarcomas of the head and neck are ‘radioresistent’ given the slow growing nature of the disease and advocate for radiotherapy in select, aggressive cases (3,28). Gripp and colleagues (29) further expanded on this advocating for radiotherapy in cases where patient is unfit for surgery or in cases of recurrence.
Radiofrequency ablation with laser photovaporization has been described to provide symptomatic relief in the palliative setting where patients are either unfit for invasive surgery and curative surgery is not possible (30).
As of this publication, all patients in our series are alive and being monitored every 6–12 months with serial imaging. Follow up times range from 2–12 years. Patients in our cohort are being followed up lifelong for disease monitoring. Interestingly our longest surviving patient is also immunocompromised having received previous renal transplant with type 2 diabetes.
This is consistent with previous literature where survival was found to be similar over 5–10-year period irrespective of site, grade or therapy. The recurrence rate was approximately 24.2% including 3 patients who underwent a total laryngectomy. Recurrence rates have been reported to be as high as 40% in the literature (6). Metastasis is rare as chondrosarcomas cause local tissue destruction before invading surrounding tissue (1). For higher grades a metastasis rate of 8.5% has been reported (2).
In our series all patients underwent debulking of tumour rather than total excision. This makes assessment of recurrence difficult. All patients in this series continue to be followed up every 6 to 12 months with serial MRI. To date, there have been no growths on interval MRIs over a period of 1–10 years.
Conclusions
LC is a slow growing tumour with a favorable prognosis. Treatment should focus on laryngeal function preservation and disease monitoring. A total laryngectomy should only be reserved in very select cases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-19-84
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-19-84). FR serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the institutional human research ethics committee (Number: 1909-02). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bathala S, Berry S, Evans RA, et al. Chondrosarcoma of larynx: Review of literature and clinical experience. J Laryngol Otol 2008;122:1127-9. [Crossref] [PubMed]
- Brandwein M, Moore S, Som P, et al. Laryngeal chondrosarcomas: a clinicopathologic study of 11 cases, including two "dedifferentiated" chondrosarcomas. Laryngoscope 1992;102:858-67. [Crossref] [PubMed]
- Coca-Pelaz A, Rodrigo JP, Triantafyllou A, et al. Chondrosarcomas of the head and neck. Eur Arch Otorhinolaryngol 2014;271:2601-9. [PubMed]
- Chin OY, Dubal PM, Sheikh AB, et al. Laryngeal chondrosarcoma: A systematic review of 592 cases. Laryngoscope 2017;127:430-9. [Crossref] [PubMed]
- Dubal PM, Svider PF, Kanumuri VV, et al. Laryngeal chondrosarcoma: A population-based analysis. Laryngoscope 2014;124:1877-81. [Crossref] [PubMed]
- Thompson LDR, Gannon FH. Chondrosarcoma of the larynx: A clinicopathologic study of 111 cases with a review of the literature. Am J Surg Pathol 2002;26:836-51. [Crossref] [PubMed]
- Rinaldo A, Howard DJ, Ferlito A. Laryngeal chondrosarcoma: A 24-year experience at the royal national throat, nose and ear hospital. Acta Oto-Laryngologica 2000;120:680-8. [Crossref] [PubMed]
- Wang Q, Chen H, Zhou S. Chondrosarcoma of the larynx: report of two cases and review of the literature. Int J Clin Exp Pathol 2015;8:2068-73. [PubMed]
- Purohit BS, Dulguerov P, Burkhardt K, et al. Dedifferentiated laryngeal chondrosarcoma: Combined morphologic and functional imaging with positron-emission tomography/magnetic resonance imaging. Laryngoscope 2014;124:E274-E277. [Crossref] [PubMed]
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO Classification of Tumours of Soft Tissue and Bone. Lyon, FRANCE: International Agency for Research on Cancer (I A R C) (UN); 2013.
- Amin MB, Edge S, Greene F, et al. editors. AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer; 2017.
- Lichtenstein L, Jaffe HL. Chondrosarcoma of Bone. Am J Pathol 1943;19:553-89. [PubMed]
- Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer 1977;40:818-31. [Crossref] [PubMed]
- Lippert BM, Rehberg E, Claassen H, et al. Laryngeal chondrosarcoma with regard to the ultrastructure. Otolaryngol Pol 2000;54:703-8. [PubMed]
- de Vincentiis M, Greco A, Fusconi M, et al. Total cricoidectomy in the treatment of laryngeal chondrosarcomas. Laryngoscope 2011;121:2375-80. [Crossref] [PubMed]
- Piazza C, Del Bon F, Grazioli P, et al. Organ preservation surgery for low- and intermediate-grade laryngeal chondrosarcomas: Analysis of 16 cases. Laryngoscope 2014;124:907-12. [Crossref] [PubMed]
- Kozelsky TF, Bonner JA, Foote RL, et al. Laryngeal chondrosarcomas: The Mayo Clinic experience. J Surg Oncol 1997;65:269-73. [Crossref] [PubMed]
- Mata JF, Leon R, Noda R, et al. Variant of partial laryngectomy in low-grade chondrosarcoma. Revista Venezolana de Oncologia 2015;27:53-6.
- Piazza C, Paderno A, Nicolai P. Conservative surgery for laryngeal chondrosarcoma: A review of the most recently proposed approaches. Curr Opin Otolaryngol Head Neck Surg 2017;25:93-100. [Crossref] [PubMed]
- Pelliccia P, Pero MMD, Mercier G, et al. Transoral endoscopic resection of low-grade, cricoid chondrosarcoma: Endoscopic management of a series of seven patients with low-grade cricoid chondrosarcoma. Ann Surg Oncol 2014;21:2767-72. [Crossref] [PubMed]
- Guthrie AJ, Chai RL. Transoral robotic surgery for the treatment of laryngeal chondrosarcoma: A case report. Am J Otolaryngol 2018;39:352-4. [Crossref] [PubMed]
- Thomé R, Thome DC, de la Cortina RA. Long-term follow-up of cartilaginous tumors of the larynx. Otolaryngol Head Neck Surg 2001;124:634-40. [PubMed]
- Rich JT, Goldstein D, Haerle SK, et al. Vascularized composite autograft for adult laryngotracheal stenosis and reconstruction. Head Neck 2016;38:253-9. [Crossref] [PubMed]
- Loos E, Meulemans J, Vranckx J, et al. Tracheal Autotransplantation for Functional Reconstruction of Extended Hemilaryngectomy Defects: A Single-Center Experience in 30 Patients. Ann Surg Oncol 2016;23:1674-83. [Crossref] [PubMed]
- Chanowski EJ, Haxer MJ, Chepeha DB. Microvascular cricoid cartilage reconstruction with the thoracodorsal artery scapular tip autogenous transplant. Laryngoscope 2012;122:282-5. [Crossref] [PubMed]
- Rovó L, Bach A, Sztano B, et al. Rotational thyrotracheopexy after cricoidectomy for low-grade laryngeal chrondrosarcoma. Laryngoscope 2017;127:1109-15. [Crossref] [PubMed]
- Ferlito A, Nicolai P, Montaguti A, et al. Chondrosarcoma of the larynx: Review of the literature and report of three cases. Am J Otolaryngol 1984;5:350-9. [Crossref] [PubMed]
- Moussavi-Harami F, Mollano A, Martin JA, et al. Intrinsic radiation resistance in human chondrosarcoma cells. Biochem Biophys Res Commun 2006;346:379-85. [Crossref] [PubMed]
- Gripp S, Pape H, Schmitt G. Chondrosarcoma of the larynx: The role of radiotherapy revisited - A case report and review of the literature. Cancer 1998;82:108-15. [Crossref] [PubMed]
- Perrot C, Cortese S, Eluecque H, et al. Laryngeal chondrosarcoma: Repeated laser and radiofrequency ablation in the palliative setting. Eur Ann Otorhinolaryngol Head Neck Dis 2013;130:91-3. [Crossref] [PubMed]
Cite this article as: Ahmadzada S, Sritharan N, Smith M, Palme CE, Riffat F. Laryngeal chondrosarcoma of the cricoid cartilage—a case series towards conservative management. Aust J Otolaryngol 2020;3:22.


