
An ex vivo comparison of over-the-counter cerumenolytics for ear wax
Introduction
Problematic ear wax (cerumen) is a common presentation to the primary care physician and is the most frequent reason for presentation for ear pain (1). Cerumen impaction is estimated to affect 6–20% of the Australian population, with higher rates observed in elderly persons and those with disability (2). The health burden of cerumen-related procedures in the United States is reported to be US $50 million, making this a significant burden on the health system (1,3). Although the total annual cost of managing cerumen in Australia is not readily available, it is likely to have similar health-related expenses because of the common lifestyles amongst the countries.
The external auditory canal is predominately self-cleaning due to specialised epithelium, glandular function and anatomy. Impaction can occur when these physiological processes fail or simply as a result of attempted removal itself (4). Impacted cerumen can be associated with symptoms of hearing loss, pain, itchiness, tinnitus, dizziness and cognitive impairment in elderly patients (1,5). Cerumen impaction can also be asymptomatic but can obstruct visualisation and assessment of the tympanic membrane, which can lead to a delay in either diagnosis or management of other otological problems (6).
The common treatment methods for clearing impacted cerumen include cerumenolytics, irrigation by syringe and microsuction. Cerumenolytics is the most common first-line management by both general practitioners (GPs) and ear, nose and throat (ENT) specialists (7). The success rate for managing cerumen impaction by irrigation has been reported to range from 68% to 92% (1). Irrigation complications can include pain, external auditory canal injury, otitis externa, vertigo and, rarely, tympanic membrane perforation (1,8). Manual removal of impacted cerumen using a binocular microscope for visualisation has a reported success rate of 90% (1) but can cause trauma to the external auditory canal, including pain, bleeding and tympanic perforation and, rarely, infection (1,9). Both methods require increased procedural and equipment costs or ongoing input from a GP with adequate training and experience and availability of equipment or input from an ENT specialist (1).
In Australia, there are many different types of commercially available ear drops. These can be separated into oil-based compounds (e.g., olive oil, CleanEars®), water-based compounds (e.g., Waxsol®, hydrogen peroxide) or non-water-/non-oil-based solutions, such as carbamide peroxide (Earclear®). Water-based cerumenolytics function by drawing water into the cerumen, resulting in its fragmentation, whereas oil-based preparations lubricate and soften the cerumen (1). Antibiotic drops are also commonly used in the outpatient setting as an adjunct infection-preventive measure. The commonly used topical antibiotics are Sofradex® and Cipro®HC. Cerumenolytic agents are commonly prescribed for managing cerumen impaction and are known to be associated with a relatively low incidence of adverse effects when used in patients without active ear infection and an intact tympanic membrane (10). Cerumenolytics reduce the need for syringing or manual removal of the impacted cerumen and can also improve the efficacy of manual removal and irrigation (1). There is little evidence to support the use of one cerumenolytic across both adult and paediatric populations (4,11). The relative success rates of different cerumenolytics for treating different cerumen consistencies are also unknown. There have been some comparisons of cerumenolytics conducted in vitro and in vivo (12-16); however, most of these investigations compared only a very small number of agents or tested some agents not commonly available in Australia.
The degree of consistency of the patient’s cerumen (hard, medium and soft) is clinically relevant to the medical practitioner. Both hard and soft cerumen are known to be a problem in the population and may require different treatments. Several studies have included consistency ratings of the cerumen (17,18); however, no investigation has directly studied whether the effectiveness of cerumenolytics is affected by cerumen consistency.
This investigation aims to compare the effectiveness of the commonly prescribed cerumenolytic agents available in Australia in softening or dissolving cerumen. We also examined whether the consistency of the cerumen influences the efficacy of each cerumenolytic agent. The hypothesis tested was that water-based cerumenolytics would be more effective than oil-based agents and that the consistency rating of the cerumen would alter the effectiveness of the cerumenolytic agent.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-20-50).
Methods
This investigation was a prospective study performed in accordance with STROBE requirements (19). This exploratory study included the analysis of cerumen samples collected from 12 patients over a 1-month period from an outpatient ENT clinic in a Queensland hospital. All patients were referred by GPs as a result of problems associated with bothersome cerumen. All patients consented to their cerumen being used and were given a patient information sheet. Patients were excluded from the study if they had concurrent ear pathology. No demographic details were recorded.
Using a Jobson Horne ear probe, cerumen was extracted manually, placed in a collection jar and stored at room temperature. Two independent observers subjectively categorised the cerumen into three different consistency ratings: soft, medium or hard (Figure 1). Cerumen analysis was performed on the same day as collection. A punch biopsy was used to standardise the size of the test sample (Figure 2). Cerumen analysis involved placing 0.5 mL of cerumenolytics with each cerumen sample. The cerumenolytics used were Waxsol®, hydrogen peroxide, Aqua Ear®, Earclear®, CleanEars®, olive oil, Cipro®HC, Sofradex®, Co-phenylcaineTM Forte and sterile water (water). Photo documentation was performed at one, two and five minutes. Test tubes were then drained of their solution, and the remaining cerumen was placed onto a sheet of paper. Outcome measures for the treated cerumen were based on a dissolving scale (ranging from 0, unchanged; 1, <25%; 2, 25–75%; and 3, >75%) and a softening scale (from one swipe with a Jobson Horne probe; ranging from 0, unchanged; 1, partially softened; and 2, completely softened). Two independent investigators rated each cerumen sample for dissolvability and softening ratings. In cases of discrepancy, the average value was recorded. The softening scale was a comparison with baseline consistency of the cerumen. Because the consistency ratings were heterogeneous, the softening scale comparisons were made within groups rather than between groups.

Data was recorded in an Excel spreadsheet. Post hoc analysis was performed on the outcome variables using the Kendall Tau-b coefficient for correlation. A Fisher’s exact test was used to compare individual agents against each other for difference in dissolvability and softening. Logistical regression analysis was performed with two independent variables (initial cerumen rating and agent) to assess their influence on the level of dissolving and softening. Stata V16.2 (StataCorp, 2019, Stata Statistical Software: Release 16, College Station, TX, USA) was used for statistical comparisons. A P value of <0.05 was considered significant. Because this was a prospective research, there were no missing data.
Ethics approval was obtained via low-risk ethics submission through the Metro South Ethics Committee. Research was performed based on the standard of Ethical Considerations in the Conduct and Reporting of Research: Privacy and Confidentiality. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This ex-vivo study did not impact on individual patient care or their future management.
Results
This exploratory study utilised a convenience sampling, which obtained wax from twelve patients which was comparable to previous studies in sample size (13,14,16). Exploratory research was conducted as a number of agents used in this study have not been investigated in prior studies. Table 1 lists the names and characteristics of the 10 cerumenolytics tested for their abilities to dissolve and soften cerumen of hard, medium and soft consistencies. The cerumenolytics fell into three categories: water-based, oil-based and non-water-/non-oil-based. Data collected included mean dissolvability and softening indices for each sample tested.
Table 1
| Categories | Brand name | Active ingredient |
|---|---|---|
| Water-based | Waxsol® | Sodium docusate |
| Aqua Ear® | Acetic acid/isopropyl alcohol solution | |
| Hydrogen peroxide | Hydrogen peroxide solution | |
| Co-phenylcaineTM Forte | Lignocaine/phenylephrine | |
| Sterile Water | Water | |
| Cipro®HC | Ciprofloxacin/hydrocortisone | |
| Sofradex® | Gramicidin/framycetin/dexamethasone | |
| Oil-based | Olive oil | Olive oil |
| CleanEars® | Mineral oil, squalane, spearmint oil | |
| Non-water-/non-oil-based (other) | Earclear® | Carbamide peroxide |
The correlation between the dissolving and softening indices of the different agents was assessed using Kendall Tau-b correlation coefficients. Values of Kendall Tau-b expressed as percentages are illustrated in Tables 2,3. These percentages could range from –100% to +100%, where zero indicated no association. This shows there are strong correlations between the individual water-based cerumenolytics (including the water-based antibiotic drops) and non-water-/non-oil-based cerumenolytics. There were also strong negative correlations between the water-based and oil-based cerumenolytics across all baseline cerumen consistency ratings, indicating an inverse relationship.
Table 2
| Agents | Waxsol® | H2O2 | Aqua Ear® | Earclear® | CleanEars® | Olive oil | Cipro®HC | Sofradex® | Co-phenyl.TM | Water |
|---|---|---|---|---|---|---|---|---|---|---|
| Waxsol® | 100% | |||||||||
| H2O2 | 0% | 100% | ||||||||
| Aqua Ear® | –62% | 36% | 100% | |||||||
| Earclear® | –6% | 11% | 39% | 100% | ||||||
| CleanEars® | –6% | 36% | –9% | –53% | 100% | |||||
| Olive oil | ||||||||||
| Cipro®HC | –9% | 29% | 33% | 60% | –52% | 100% | ||||
| Sofradex® | 28% | 3% | 18% | 48% | –49% | 54% | 100% | |||
| Co-phenyl.TM | –8% | 2% | 13% | 72% | –42% | 42% | 35% | 100% | ||
| Water | 25% | –5% | 5% | 63% | –37% | 21% | 52% | 70% | 100% |
*, Correlation value is represented as a percentage for Kendall Tau-b coefficient. Correlations between 0% and 25% are yellow and 50% and 99% are green.
Table 3
| Agents | Waxsol® | H2O2 | Aqua Ear® | Earclear® | CleanEars® | Olive oil | Cipro®HC | Sofradex® | Co-phenyl.TM | Water |
|---|---|---|---|---|---|---|---|---|---|---|
| Waxsol® | 100% | |||||||||
| H2O2 | 34% | 100% | ||||||||
| Aqua Ear® | –31% | –21% | 100% | |||||||
| Earclear® | 38% | 38% | 35% | 100% | ||||||
| CleanEars® | 84% | 19% | –12% | 25% | 100% | |||||
| Olive oil | 68% | 54% | –29% | 12% | 82% | 100% | ||||
| Cipro®HC | –37% | –37% | –31% | –65% | –60% | –49% | 100% | |||
| Sofradex® | 12% | 6% | –84% | –20% | –13% | 0% | 48% | 100% | ||
| Co-phenyl.TM | –8% | –3% | 8% | 4% | –16% | –26% | –8% | –16% | 100% | |
| Water | –36% | –28% | –25% | –24% | –43% | –52% | 25% | 21% | 67% | 100% |
*, Correlation value is represented as a percentage for Kendall Tau-b coefficient. Correlations between 0% and 25% are yellow and 50% and 99% are green.
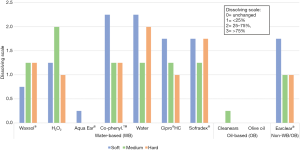
As illustrated by Figures 3,4 water-based agents (with the exception of Aqua Ear®) had greater mean dissolvability and softening indices compared with oil-based agents. The only non-water-/non-oil-based agent Earclear® dissolved and softened cerumen to the same extent as water-based agents.

Waxsol® is the number one selling product on the market in Australia (20). A Fisher’s exact test was performed comparing each agent to Waxsol® in terms of dissolving and softening indices. A statistically significant difference in the dissolving index was noted comparing Waxsol® to Aqua Ear® (P=0.00), CleanEars® (P=0.00) and olive oil (P=0.00). There was no statistical difference in the dissolving index between Waxsol® and other agents. A Fisher’s exact test was performed on the softening index comparing Waxsol® to the other agents across all baseline cerumen consistencies, and a statistically significant difference was demonstrated for Aqua Ear® (P=0.005), CleanEars® (P=0.001) and olive oil (P=0.00). There was no statistically significant difference between Waxsol® and other agents for the softening index across all baseline cerumen consistency ratings.
Water was the most efficacious agent used (Figures 3,4). A Fisher’s exact test was performed for water (most cost-effective and potentially most efficacious) agent comparing it with the other agents in terms of both dissolving and softening abilities. A statistically significant difference was noted for Waxsol® (P=0.016), Aqua Ear® (P=0.00), CleanEars® (P=0.000) and olive oil (P=0.00) in dissolving index. A Fisher’s exact test was performed on the softening index comparing water to the other agents across all baseline cerumen consistencies, and a statistically significant difference was found for Waxsol® (P=0.27), Aqua Ear® (P=0.00), CleanEars® (P=0.000), olive oil (P=0.00), Cipro®HC HC (P=0.027) and Sofradex® (P=0.009).
Logistical regression was used for further analysis. Our dependent variable was the dissolving index (<25%, ≥25%), and independent variables of initial cerumen rating and agent were used to assess their influence on the level of dissolvability. When examining water and Waxsol®, water was more likely to result in a dissolving level ≥25% (AdjR2 =0.3832, P=0.011). We also assessed the influence on the softening index using a logistical regression with two independent variables (initial cerumen rating and agent) and a single dependent variable (softening index). Water was 26 times more likely than Waxsol® to completely soften the cerumen, which was statistically significant (AdjR2 =0.3716, P=0.018). Because this was an exploratory analysis, no corrections were made for multiple hypothesis testing.
Discussion
In Australia, there is a growing number of cerumenolytic agents currently on the market used for treating bothersome cerumen impaction. The interest in over-the-counter cerumenolytics has remained steady because of patient comfort and the relative cost of microsuction therapies. The 2017 American Academy of Otolaryngology guideline on cerumen stated that ‘there are a limited number of well controlled, high quality, homogenous studies demonstrating the efficacy of topical agents’ (1).
It is widely accepted in the available literature that treatment with any form of water- or oil-based cerumenolytic is more effective than no treatment (21). A Cochrane review of the published evidence for cerumenolytics concluded that cerumenolytic drops were effective, but the authors of the study did not find any difference amongst agents (11).
The current investigation used a methodology similar to previous research in this area, with key adjustments made to more accurately assess the effectiveness of cerumenolytics and to replicate real-life application. Cerumen size and volume of the solution were standardised to improve consistency of results. Although Srisukhumchai et al. [2019] combined cerumen samples from multiple patients, which potentially confounded the interpretation of results (16), we performed a separate investigation of patient samples. Our study also used a greater variety of commonly used cerumenolytics available in Australia, some of which had not been previously tested. No known studies have examined Cipro®HC, Sofradex® or Co-phenylcaineTM Forte, which are commonly used treatments.
A 5-minute period for evaluating the effect of cerumenolytics was used deliberately by examiners as it was felt that this reflected the physiological time frame in which most ear drops would be instilled in a patient’s ear. There is no consensus in the literature on a time period to determine cerumenolytic effect. Srisukhumchai et al. [2019] used a period of 60 minutes, Saxby et al. [2013] assessed the effect after 12 hours and Whatley et al. [2003] assessed the cerumenolytic effect after 15 minutes (14,16,18).
Other similar studies have examined the degree of cerumen disintegration but did not use a softening scale. This was considered an important measure because of the different proposed cerumenolytic mechanisms of water- and oil-based drops. Water-based preparations are thought to draw in water via osmosis and fragment the cerumen, whereas oil-based agents lubricate and soften the cerumen without fragmentation (1).
This investigation examined the effect of different cerumenolytics against cerumen of various consistencies. The results of this study showed a similar effectiveness and no statistically significant difference with agents against soft, medium and hard cerumen consistencies. To our knowledge, no other prior studies have compared this variable.
Across the three broad categories of agents, non-water-/non-oil-based agents were comparable with water-based products, and oil-based cerumenolytics had limited effect. The results of this study indicate that water-based cerumenolytics were more effective than oil-based cerumenolytics. Some studies have reported similar results (13,14). However, there is no consensus in the literature; some studies reported no significant difference between water-based and non-water-based agents, which was the same finding of the Cochrane review (11,21).
Olive oil is readily available in the home environment and is still commonly used for treating impacted cerumen. In our study, oil-based agents, especially olive oil, were ineffective cerumenolytics. Similarly, Saxby et al. [2013] reported that oil-based products were relatively ineffective, a result Chalishazar et al. [2007] also found with the added conclusion that olive oil was totally ineffective (13,14).
Sterile water is an inexpensive, readily available cerumenolytic agent. In our study, sterile water alone had higher dissolving and softening indices than all other agents studied. Saxby et al. [2013] also found that distilled water was associated with the greatest degree of cerumenolysis (14). A recent Cochrane review found no evidence that using water alone was better or worse than commercial cerumenolytic products (11). However, the prolonged use of water in the external ear canal may predispose the patient to otitis externa (2).
In this study, water was more effective than Waxsol® in softening and dissolving cerumen, and this difference was statistically significant. According to the IMS Health Australia Pharmaceutical Index (July 2018), Waxsol® was the best-selling product (20). The effectiveness of sodium docusate (the active ingredient in Waxsol®) has been described in three previous studies. One in vitro study found substantial cerumen disintegration by Waxsol® (12). Another in vitro study that compared sodium docusate to sodium bicarbonate found superior results with sodium bicarbonate (16). An in vivo randomised controlled trial compared docusate sodium to triethanolamine polypeptide (Cerumenex®) and found that docusate sodium solution was a more effective cerumenolytic than Cerumenex®; however, this finding has little relevance to Australian practitioners, because Cerumenex® is not commonly available in Australian pharmacies (15). Aqua Ear® was the least effective water-based agent in our research, but its primary prescribed function is for the treatment of inflammation and infection, rather than cerumenolysis.
Excluding sterile water, when evaluating the other water-based agents, there was no statistically significant difference observed. There is similar effectiveness of the other water-based preparations and more readily available agents included Waxsol® and hydrogen peroxide.
A limitation of our study was the sample size. However, the number of samples was comparable with that of other in vitro/ex vivo studies in this field. In some of these published studies, the cerumen was pooled and mixed, and the researchers made no attempt to subclassify cerumen consistencies (14,16). Future studies using larger patient numbers would provide more statistically robust results. The current investigation was an ex vivo study; therefore, the major conclusions need to be validated in clinical in vivo trials and potential complications associated with each agent should be evaluated.
Conclusions
Cerumen impaction is a common and significant health problem. This study provides an Australian-specific, up-to-date trial comparing the effectiveness of commonly available cerumenolytics in Australian pharmacies. There is a lack of consensus in the literature on the most appropriate cerumenolytic, and in many instances, the choice of cerumenolytic is based on the practitioner’s individual experience. Oil-based cerumenolytics are still commonly prescribed by GPs and ENT specialists. Adding to the growing evidence base, we found that oil-based agents, more specifically, olive oil, were ineffective cerumenolytics. Water-based cerumenolytics were the most effective across all consistencies.
While there are a growing number of over-the-counter, relatively expensive agents on the market, none in this study were more effective than sterile water. However, due to potential increased risk of otitis externa, further in-vivo studies are required. The authors have concluded for everyday practice, oil-based agents should be avoided and any of the water-based cerumenolytics, including Waxsol® and hydrogen peroxide, would be the next best treatment regardless of cerumen consistency.
Acknowledgments
The authors acknowledge the Metro South Health Centres for Health Research for facilitation of the Metro South Health Biostatistics Service provided by the Queensland Facility for Advanced Bioinformatics (QFAB) and funded by the Metro South Study, Education and Research Trust Account (SERTA).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-20-50
Peer Review File: Available at http://dx.doi.org/10.21037/ajo-20-50
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-20-50). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the research are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Metro South Ethics Committee (HREC/2020/QMS/61086) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical Practice Guideline (Update): Earwax (Cerumen Impaction). Otolaryngol Head Neck Surg 2017;156:S1-29. [Crossref] [PubMed]
- Straw S. Submission 25. In: Inquiry into the Hearing Health and Wellbeing of Australia. Glen Waverley: Crystal Clear Ear Clinic, 2016.
- Yang EL, Macy TM, Wang KH, et al. Economic and Demographic Characteristics of Cerumen Extraction Claims to Medicare. JAMA Otolaryngol Head Neck Surg 2016;142:157-61. [Crossref] [PubMed]
- Wright T. Ear wax. BMJ 2015;351:h3601. [Crossref] [PubMed]
- Moore AM, Voytas J, Kowalski D, et al. Cerumen, hearing, and cognition in the elderly. J Am Med Dir Assoc 2002;3:136-9. [Crossref] [PubMed]
- Browning GG. Ear wax. BMJ Clin Evid 2008;2008:0504.
- Poulton S, Yau S, Anderson D, et al. Ear wax management. Aust Fam Physician 2015;44:731-4. [PubMed]
- Pavlidis C, Pickering JA. Water as a fast acting wax softening agent before ear syringing. Aust Fam Physician 2005;34:303-4. [PubMed]
- Snelling JD, Smithard A, Waddell A. Noise levels generated within the external auditory canal during microsuction aural toilet and the effect on hearing: a prospective controlled series. Clin Otolaryngol 2009;34:21-5. [Crossref] [PubMed]
- Roland PS, Smith TL, Schwartz SR, et al. Clinical practice guideline: cerumen impaction. Otolaryngol Head Neck Surg 2008;139:S1-21. [Crossref] [PubMed]
- Aaron K, Cooper TE, Warner L, et al. Ear drops for the removal of ear wax. Cochrane Database Syst Rev 2018;7:CD012171. [PubMed]
- Bellini MJ, Terry RM, Lewis FA. An evaluation of common cerumenolytic agents: an in-vitro study. Clin Otolaryngol Allied Sci 1989;14:23-5. [Crossref] [PubMed]
- Chalishazar U, Williams H. Back to basics: finding an optimal cerumenolytic (earwax solvent). Br J Nurs 2007;16:806-8. [Crossref] [PubMed]
- Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol 2013;127:1067-70. [Crossref] [PubMed]
- Singer AJ, Sauris E, Viccellio AW. Ceruminolytic effects of docusate sodium: a randomized, controlled trial. Ann Emerg Med 2000;36:228-32. [Crossref] [PubMed]
- Srisukhumchai C, Kasemsiri P, Rattanaanekchai T, et al. A comparative in vitro study on the cerumenolytic effect of docusate sodium versus 2.5% sodium bicarbonate using UV-visible absorption spectroscopy. J Otol 2020;15:99-102. [Crossref] [PubMed]
- Propst EJ, George T, Janjua A, et al. Removal of impacted cerumen in children using an aural irrigation system. Int J Pediatr Otorhinolaryngol 2012;76:1840-3. [Crossref] [PubMed]
- Whatley VN, Dodds CL, Paul RI. Randomized clinical trial of docusate, triethanolamine polypeptide, and irrigation in cerumen removal in children. Arch Pediatr Adolesc Med 2003;157:1177-80. [Crossref] [PubMed]
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019;13:S31-4. [Crossref] [PubMed]
- Australian Pharmaceutical Audit, S02 OTHER OTOLOGICALS. IQVIA Solutions Ltd., 2018.
- Clegg AJ, Loveman E, Gospodarevskaya E, et al. The safety and effectiveness of different methods of earwax removal: a systematic review and economic evaluation. Health Technol Assess 2010;14:1-192. [Crossref] [PubMed]
Cite this article as: Tynan T, Griffin A, Whitfield BCS. An ex vivo comparison of over-the-counter cerumenolytics for ear wax. Aust J Otolaryngol 2020;3:33.


