Change in swallow function after supraglottoplasty
Introduction
Laryngomalacia is the most common cause of stridor in infants and is the most common congenital anomaly of the larynx (1). It is thought to be caused by poor neurologic tone and integration (2,3). The severity of laryngomalacia varies, but most patients are managed conservatively with only 10–30% requiring surgical intervention, which is almost always supraglottoplasty (1-4).
In patients with laryngomalacia severe enough to require surgical intervention, subjective feeding difficulty is present most of the time (4,5). Three recent studies have looked at frequency of objectively measured dysphagia in patients with laryngomalacia and found disparate rates ranging from 14–88% (6-8). Studies have also reported disparate rates of new dysphagia after supraglottoplasty ranging from 0 to 28% (7,9-11). These wide ranges are partially due to variations in inclusion criteria and definitions of dysphagia and perhaps random variation due to small sample sizes.
Research has shown greater parent-reported emotional impact of laryngomalacia when there was associated aspiration or penetration on FEES (6). This suggests that, in addition to the inherent benefit to the child to have normal swallow function, postoperative swallow function is important to parents as well and thus is important to discuss preoperatively.
To our knowledge, there is no published literature comparing pre- and postoperative diet and VFSS data that includes all patients undergoing supraglottoplasty at a single center. Previous studies have excluded patients without preoperative objective testing or patients with known preoperative dysphagia. The objective of this study was to describe change in diet and swallow function in patients with laryngomalacia after supraglottoplasty. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-20-70).
Methods
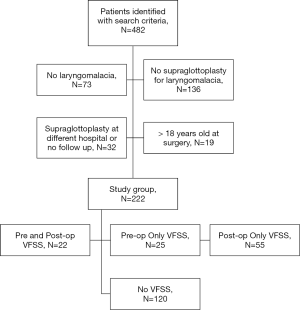
We obtained approval from the University of Michigan Institutional Review Board to perform a retrospective chart review of pediatric patients undergoing surgical treatment of laryngomalacia from 1992 through 2015 (HUM00110934). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Electronic Medical Record Search Engine (EMERSE) (12) was used to identify patients with the keyword “laryngomalacia”. Once all patient medical records with the above keyword were identified, their charts were reviewed to determine eligibility. Patients were excluded if their first surgery for laryngomalacia was performed at a different hospital or if there was no postoperative follow up.
The intervention was supraglottoplasty. Surgical techniques included trimming of redundant arytenoid tissue and/or releasing of the aryepiglottic folds using cold steel, microdebrider, or laser ablation as well as suture suspension of the epiglottis. The exact technique chosen was at the discretion of the surgeon. The primary outcome was change in diet. Diet was determined by guardian report when a video fluoroscopic swallow study (VFSS) was not performed. Guardian report was oral, not part of a questionnaire. Change in diet was defined as increase or decrease in dietary restrictions regarding consistency of liquid patient was able to tolerate. Ability to tolerate a specific consistency was determined objectively by VFSS when available. When VFSS was not performed, ability to tolerate different liquid consistencies was determined by clinical, bedside assessment.
Dysphagia was determined by VFSS, which is synonymous with 3-phase swallow study, or modified barium study (MBS). Dysphagia was categorized as mild, moderate, or severe primarily based on the degree to which the dysphagia impacted the child’s ability to meet nutritional needs orally. A mild impairment required some degree of diet or positioning modification to enable the child to continue meeting nutritional needs orally. Moderate impairment required supplemental tube feedings for a specific consistency (usually liquids), but the child could still eat a significant amount orally. Severe impairment required the patient be nil per os (NPO) or receive therapeutic tastes only. These determinations were made by an experienced speech language pathologist.
VFSS was performed in the radiology department with a radiologist and speech pathologist or occupational therapist present. Patients were positioned in an age-appropriate tumbleform chair and fed various consistencies of liquid, and when appropriate solid, food mixed with barium. Swallowing was assessed in the anterior-posterior and lateral planes. The presence or absence of aspiration, penetration, and abnormal coordination was documented.
Comorbidities, both medical and surgical, were determined by chart review. Preoperative diet and VFSS were those immediately prior to supraglottoplasty. Each included preoperative VFSS was performed after another airway surgery, but prior to supraglottoplasty. Postoperative VFSS, particularly last postoperative VFSS may have been performed after another airway surgery.
Statistical analyses were performed using Statistical Packages for Social Sciences (SPSS) version 14 (New York, USA). T test, chi square, Fisher’s exact and ANOVA test were used as appropriate. Multiple linear logistic regression and survival analysis were used for multi variable analysis. A P value less than 0.05 was considered statistically significant.
Results
EMERSE identified 482 patients meeting our search criteria. After chart review, 222 patients were included in our study. The inclusion and exclusion criteria are detailed in Figure 1. There were 98 (44%) females and 124 (56%) males with a median age at surgical treatment of 7 months (range: 3 days–17 years old). Surgical details and complications are detailed in Table 1.

Full table
Presenting symptom
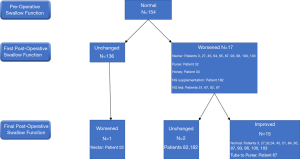
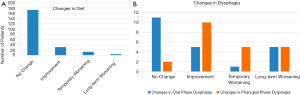
Respiratory distress of variable severity was the primary presenting symptom in all but 1 patient, for whom dysphagia was their primary presenting symptom. Sixty-one (27%) patients had preoperative parent-reported feeding difficulty associated with their respiratory distress. Sixty-eight (38%) patients had pre-operative diet modifications (Figure 2) for reasons that were often multifactorial and not directly attributable to the patient’s laryngomalacia.
Diet restrictions
Most patients had no diet restrictions prior to surgery (n=156, 70%). Immediately (within the first week) after surgery the changes in swallowing function is shown in Figure 3 for those with normal, and Figure 4 for those with abnormal swallowing function. The median dysphagia duration is 165 days (range: 6–1,825 days).
There is a strong association with pre-operative diet modification (P=0.00), presence of laryngeal cleft (P=0.00), previous CTR (P=0.011), history of cardiac surgery (P=0.001), association with syndrome (P=0.00), presence of oral aversion (P=0.00), pre-operative NGT (P=0.00) and G tube (P=0.00), pre-operative swallowing dysfunction (P=0.000) with dysphagia.
In patients with a normal function (Figure 4), the dysphagia resolved (back to normal diet) in 14 of 17 (82%) patients over a median of 30 days (range: 19–720 days).
Videofluoroscopy & swallowing function
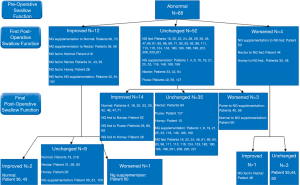
Pre- and post-operative VFSS result is shown in Figure 5. Forty-seven patients (21%) had preoperative VFSS for review. There were 22 (10%) with both pre- and postoperative VFSS results to allow determination of change in VFSS after supraglottoplasty. For those who had post-operative VFSS, this was done on a median of day 12 (range: 1–3,149 days). Thirty patients had the first post-operative VFSS done less than a week post-operatively. These patients were identified by their pre-operative assessment as high risk or struggled with feeding in the immediate post-operative period.
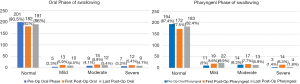
Five of these 22 patients (23%) had a temporary increase in severity of pharyngeal phase dysphagia after surgery that resolved over a median of 5 months (range: 6 days–5 years). Another 5 had a persistent worsening of pharyngeal phase dysphagia at last follow up over a median of 12 months (range: 4 months–3 years). And 45% of patients had an immediate improvement in pharyngeal dysphagia postoperatively (Figure 2). Median length of follow up for those patients with persistent pharyngeal phase dysphagia was one year (range: 4 months–3 years).
Interestingly, oral phase dysphagia was altered in 50% (11 patients) in whom pre- and post-operative VFSS was obtained. Five of these 22 patients had an improvement in oral phase dysphagia, 1 had a temporary worsening, and 5 had persistent worsening in oral phase dysphagia at last follow up (Figure 2).
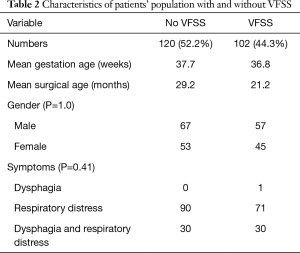
Table 2 summarises the characteristics of patients with and without VFSS. There were no significant differences for the most part. The difference in the supraglottoplasty technique is significant for the use with laser (P=0.00) and microdebrider (P=0.00), with more of these methods being used for the patients with VFSS. Unsurprisingly, more patients who had VFSS had oral aversion (P=0.00) and pre-operative NGT (P=0.00). Pre-operative gastrostomy tube (P=0.28) however, was evenly distributed between the two.
Full table
Using multiple linear regression analysis, no comorbidity or surgical technique was associated with change in dysphagia or diet restriction in a statistically significant way and therefore are not further displayed.
Discussion
The primary focus before and after treatment of laryngomalacia with supraglottoplasty tends to be airway centric. A vital component to the child’s well-being and overall quality of life includes swallowing function and nutrition. The major finding of this study was that after supraglottoplasty 6% of patients had temporary increase in diet restrictions, and 2% of patients had persistent increase in diet restrictions at last follow up. Worsening in oral and pharyngeal phase dysphagia for some length of time was observed in 27% and 45% of patients who underwent both pre- and postoperative VFSS, respectively.
Postoperative diet restrictions have previously been reported by Eustaquio et al. and Anderson de Moreno et al. as 4% and 3%, respectively, which are comparable to our 8% rate (9,11). These studies did not discuss whether these new postoperative diet restrictions were temporary or persistent and excluded patients with preoperative dysphagia, which the current study does not.
Pre- and postoperative objective swallow assessments have been previously studied by Richter et al. (7). They performed FEES on 50 patients and found no worsening of dysphagia postoperatively. In contrast to the current study, they observed an 88% preoperative penetration rate with 81% improvement in penetration at median 3.8 months postoperatively. The current study may have identified more cases of temporary worsening of dysphagia due to testing earlier than 3.8 months postoperatively, but this does not explain the 23% rate of long-term worsening of pharyngeal dysphagia in the current study.
This study’s rate of abnormal preoperative VFSS was 68%, which is comparable to that of Simons et al. and Richter et al., who reported 75.7% and 88% of baseline objective swallow evaluations were abnormal, respectively (1,8). There is significant selection bias in these results as only 21% of this study’s cohort and 57% of Simons’ cohort underwent objective swallow evaluation, with only 10% (n=22) underwent both pre- and post-operative VFSS. In our study cohort, we expect the decision to obtain preoperative VFSS was driven by clinical concern for dysphagia, and therefore the authors suspect that if all 222 patients underwent preoperative VFSS, the rate of abnormal VFSS would be lower. This highlights an area for future research to better understand how swallow objectively changes after supraglottoplasty.
In our study population, 67% had other co-morbidities with 21% neurocognitive disorders, 13% genetic abnormalities, 13% congenital cardiovascular diseases and 8% oral aversion, increasing the risk of pre-existing dysphagia. In addition, 38% had concomitant airway diagnosis, and 21% had previous airway surgery, all of which may impact on the 14% of cases that required revision surgery and the 4 requiring tracheostomy, reflecting either severe disease and or confounding airway condition resulting in persistent airway obstruction. These may also impact on the pre- and post-operative dysphagia. It is important to identify these factors to counsel the parents, identify, prognosticate and manage dysphagia that may persist.
With respect to GERD, 71% of patients in this study were treated for it postoperatively, which is within the range of GERD treatment reported in the literature for patients with laryngomalacia (2,3,6,8). Similarly, the rate of concomitant airway diagnoses in this study was comparable to previous studies (8). Our cohort had higher rates of congenital cardiovascular and neurologic comorbid conditions than previous studies (8). This may be due to our entire cohort having laryngomalacia severe enough to warrant surgical intervention as compared with some prior studies including all patients with laryngomalacia. However, a previous study did not identify a statistically significant correlation between severity of laryngomalacia and comorbidities (8). While comorbidities are often discussed as risk factors for postoperative dysphagia, only preoperative dysphagia and prematurity have been proven to correlate with postoperative dysphagia (11).
When considering the demographics and comorbid conditions of the 4 patients who had long-term worsening in diet restriction, one was mildly preterm (35 weeks’ gestation). One had cerebral palsy. Age range was 2 months to 15 months old at time of surgery. Carbon dioxide laser alone or in combination with microdebrider or cold steel was used during each surgery. Each of these 4 patients had a different surgeon. No patient had any other airway surgery. Notably, 3 out of 4 had additional airway diagnoses (unilateral vocal cord paresis, tracheomalacia, and bronchomalacia). Only one had preoperative VFSS and subsequent preoperative diet modification to thickened liquids.
The 2% rate of long-term increased diet restriction observed in this study is important to consider. Worsened dysphagia is cited as a reason not to perform supraglottoplasty and previous studies looking at large numbers of patients did not describe time of increased diet restrictions. Despite this small risk of long-term worsened dysphagia, this data can be useful when counselling families. While it should not deter from intervention, it is likely useful in optimally consenting families of the rare possibility of long-term dysphagia. Prospective analysis and additional studies are needed to better understand the mechanisms underlying dysphagia after supraglottoplasty. It is interesting that 75% of the patients in this study with long term increased diet restrictions had additional airway diagnoses compared with 38% of the overall study group. This is an area of particular interest for future prospective studies.
There are limitations to the current study that must be considered. First, this study is dependent on chart review, and therefore no causality can be determined between supraglottoplasty and change in diet or dysphagia. Second, the exact supraglottoplasty technique were heterogenous; although, technique has not been shown to affect dysphagia rates in previous study (13). This retrospective, 39 patient cohort study by Rastatter et al. was performed to directly compare postoperative dysphagia after laser versus cold steel supraglottoplasty after a prior study identified a 37% rate of dysphagia after laser supraglottoplasty without any comparison (13). Rastatter et al. observed new onset dysphagia in 31% of patients who underwent cold steel supraglottoplasty and 56% who underwent laser supraglottoplasty with P=0.18 (13). Third, data available were not formatted for the intention of ex post facto analysis. This is especially important when considering the clinical significance of our VFSS results, which represent a small, non-random subset of our study population. Finally, the sample size is small hence the results need to be interpreted with caution.
Specifically, most of our patients did not have preoperative and/or postoperative VFSS, limiting ability to draw conclusions regarding objective change in dysphagia. Additionally, more patients underwent postoperative VFSS than preoperative, which may have resulted in diet restrictions that would have been instituted preoperatively if a preoperative VFSS had been performed. Another limitation is that severity of laryngomalacia and location of malacia within the supraglottis was not routinely or uniformly documented. This precluded analysis of association of dysphagia with severity and location of malacia, which have been associated in previous studies (4-6).
Drawing definitive conclusions from this study’s oral phase dysphagia assessments poses challenges that we plan to elucidate in prospective analysis. First, the mechanism by which supraglottoplasty could directly impact oral phase swallow function is not obvious. Second, both worsening and improvement was observed at equal rates. These results are most likely indicative of the complex, multifactorial nature of swallow function and changing variables other than the effect of supraglottoplasty during the study period. For example, with time and speech and swallow therapy, dysphagia should improve in most patients; however, improved cooperation with objective swallow assessments may unmask dysphagia more severe than previously thought when assessment was more limited.
These limitations inform opportunities for future research including prospective, objective comparison or pre- and postoperative swallow function in a study group representative of the patient population undergoing supraglottoplasty. Pending more robust data from which to draw conclusions, it is important for families to be informed regarding the possibility of dysphagia, either transient or long-term, after supraglottoplasty, particularly since no comorbidities were identified as correlating with worse postoperative dysphagia in this study.
Conclusions
Supraglottoplasty is associated with reduction in diet restrictions in less than half of patients with preoperative diet restrictions. Transient and long-term increase in diet restrictions with associated worsening dysphagia occurs in a small subset of patients after supraglottoplasty. This occurs in patients with and without preoperative diet restrictions. Preoperative and postoperative objective assessment of swallow function should be considered for all patients undergoing supraglottoplasty.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-20-70
Peer Review File: Available at http://dx.doi.org/10.21037/ajo-20-70
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-20-70). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Michigan Institutional Review Board (HUM00110934) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Richter GT, Thompson DM. The surgical management of laryngomalacia. Otolaryngol Clin North Am 2008;41:837-64. vii. [Crossref] [PubMed]
- Thorne MC, Garetz SL. Laryngomalacia: Review and Summary of Current Clinical Practice in 2015. Paediatr Respir Rev 2016;17:3-8. [Crossref] [PubMed]
- Thompson DM. Abnormal sensorimotor integrative function of the larynx in congenital laryngomalacia: a new theory of etiology. Laryngoscope 2007;117:1-33. [Crossref] [PubMed]
- Landry AM, Thompson DM. Laryngomalacia: disease presentation, spectrum, and management. Int J Pediatr 2012;2012:753526. [Crossref] [PubMed]
- Kilpatrick LA, Boyette JR, Hartzell LD, et al. Prospective quality of life assessment in congenital laryngomalacia. Int J Pediatr Otorhinolaryngol 2014;78:583-7. [Crossref] [PubMed]
- Thottam PJ, Simons JP, Choi S, et al. Clinical relevance of quality of life in laryngomalacia. Laryngoscope 2016;126:1232-5. [Crossref] [PubMed]
- Richter GT, Wootten CT, Rutter MJ, et al. Impact of supraglottoplasty on aspiration in severe laryngomalacia. Ann Otol Rhinol Laryngol 2009;118:259-66. [Crossref] [PubMed]
- Simons JP, Greenberg LL, Mehta DK, et al. Laryngomalacia and swallowing function in children. Laryngoscope 2016;126:478-84. [Crossref] [PubMed]
- Anderson de Moreno LC, Matt BH. The effects of prematurity on incidence of aspiration following supraglottoplasty for laryngomalacia. Laryngoscope 2014;124:777-80. [Crossref] [PubMed]
- Eustaquio M, Lee EN, Digoy GP. Feeding outcomes in infants after supraglottoplasty. Otolaryngol Head Neck Surg 2011;145:818-22. [Crossref] [PubMed]
- Schroeder JW Jr, Thakkar KH, Poznanovic SA, et al. Aspiration following CO(2) laser-assisted supraglottoplasty. Int J Pediatr Otorhinolaryngol 2008;72:985-90. [Crossref] [PubMed]
- Hanauer DA, Mei Q, Law J, et al. Supporting information retrieval from electronic health records: A report of University of Michigan's nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE). J Biomed Inform 2015;55:290-300. [Crossref] [PubMed]
- Rastatter JC, Schroeder JW, Hoff SR, et al. Aspiration before and after Supraglottoplasty regardless of Technique. Int J Otolaryngol 2010;2010:912814. [Crossref] [PubMed]
Cite this article as: Wertz A, Ha JF, Driver LE, Zopf DA. Change in swallow function after supraglottoplasty. Aust J Otolaryngol 2021;4:3.