
Radiofrequency ablation in the treatment of inferior turbinate hypertrophy
Introduction
Nasal obstruction is the cardinal symptom present in a collection of medical conditions that implies insufficient airflow through the nose (1). One of the most common causes of chronic nasal obstruction is inferior turbinate hypertrophy (ITH), a pathology that occurs secondary to a number of triggering factors such as allergic and non-allergic rhinitis, sinusitis, congenital variations and environmental factors (2). In 2017–2018 an estimated 19% of Australians (1 in 5) suffered with allergic rhinitis and in 2012, approximately 1.9 million Australians were diagnosed with chronic rhinosinusitis (3,4), which was higher than the number of Australians diagnosed with heart failure or back pain. Initial management of nasal obstruction is often conservative, using pharmacological treatments including nasal glucocorticoid sprays, oral steroids, antihistamines and decongestants (5). However, in cases of structural abnormalities such as turbinate hypertrophy, medical treatment only works temporarily and surgical intervention is often the next progression (5). Since the 1890’s, a multitude of surgical interventions have been implemented to tackle this pathology with procedures such as electrocautery, laser cautery and turbinectomy being used effectively to this day (6-9). Any surgical procedure, however, comes with direct and indirect costs such as the cost of theatre time and equipment used as well as indirect costs such as absence from work. There are also complications, and more invasive surgeries may result in bleeding, crusting, synechiae, atrophy and subsequent nasal dryness (6,9,10). As a result of the multitude of options, there has been no clear technique that is preferred as the optimal treatment modality. This is partially due to the lack of long term prospective studies comparing the procedures (11).
Radiofrequency ablation (RFA) is a technique that was developed in 1998 at Stanford University in the US and is being used and recognised as a minimally invasive and effective mode of treatment when compared to surgery (12-15). RFA uses radiofrequency waves to deliver energy to tissue 2–4 mm depth from the electrode head, subsequently denaturing the tissue proteins in the deep mucosa whilst preserving the surface tissue (16). The ablated tissue then scars and shrinks with time resulting in reduced nasal blockage and improving patient symptoms (13,17,18).
Currently the treatment is incorporated into the National Institute for Health and Care Excellence (NICE) guidelines for turbinate hypertrophy in the UK and is often used in patients prior to considering more invasive surgery (19). In the US, the College of Otolaryngology and Head and Neck Surgery offers RFA as an evidenced and effective treatment option in patients with allergic rhinitis, and it has subsequently become one of the two most common procedures offered in the US for this condition (19,20). RFA has been offered at the Royal Brisbane and Women’s Hospital in Brisbane since 2016 and at Ipswich Hospital in Queensland since 2017, however, to our knowledge, it is not actively offered in other Australian tertiary hospitals, despite significant evidence to indicate its effectivity and use in other parts of the world. Although treatments can be quantitatively assessed for turbinate hypertrophy (21), quality of life (QOL) measures appear to offer a more clinically applicable and practically useful information with regards to outcomes and implementation. This paper evaluates the subjective efficacy of RFA using the well-established Sino-Nasal Outcome Test (SNOT 22) in 98 patients with chronic nasal obstruction over a period of 6 months in the ENT clinic at the Royal Brisbane and Women’s Hospital Brisbane to assess its validity as an alternative to surgical intervention. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-20-52).
Methods
General approach
This is a retrospective, pre-post intervention, observational analysis that observed and compared the symptomatic efficacy of RFA in patients with ITH at baseline and then 1 month and 6 months post RFA treatment. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Royal Brisbane and Women’s Hospital Human Research Ethics Committee under reference LNR/2018/QRBW/46297 and individual consent for this retrospective analysis was waived. The Funding code is 41689.
Population
From January 2015 to June 2017, 189 adult participants (100 male, 89 female) with chronic nasal obstruction secondary to suspected ITH, refractory to previous medical management, were managed with RFA at this institution. From this sample, 98 participants completed the preoperative (baseline) and at least one postoperative SNOT 22 score and were included in the SNOT 22 analysis. All participants who progressed to RFA treatment had experienced symptoms for at least 6 months and had trialled at least one method of pharmacological treatment that included intranasal corticosteroids (INCS). The assessment of these participants was predominantly based on subjective and symptomatic concerns of the patient, and clinician’s assessment of suitability. Selection criteria included the following: bilateral or side changing nasal blockage in the absence of pronounced septal deviation, obvious nasal polyps and failed treatment with medical management.
Exclusion criteria
Patients not offered RFA were those who exhibited symptoms of unilateral nasal blockage and evidence of nasal polyps. Bilateral nasal obstruction appears to have a greater correlation of ITH (22,23). Participants who could not understand or converse in English were not asked to complete SNOT surveys and hence did not have their data analysed. Participants who scored less than nine on their initial overall SNOT 22 survey were excluded from the SNOT 22 analysis. Participants who did not complete the preoperative SNOT 22 survey or at least one postoperative SNOT 22 survey were not included in the SNOT 22 analysis.
Evaluation
For this study, participants were required to complete the SNOT 22 survey during visits at the clinic at enrolment (baseline) and then 1-month and 6 months post procedure. The Sino-Nasal Outcome Test is a 22-item (SNOT 22) outcome measure that has been validated as an effective measure of QOL and surgical outcomes in participants with chronic sino nasal disease (©2006, Washington University, St. Louis, MO, USA) (24-26). Initially it was a 20-question tool; it was expanded to 22 questions to provide a greater applicability and an accurate clinical reflection (24,26). The SNOT 22 uses a scale which grades responses as 0= “no problem”, 1= “very mild problem”, 2= “mild or slight problem”, 3= “moderate problem”, 4= “severe problem”, and 5= “problem as bad as it can be” with a total score ranging from 0–110 for each patient (27). An example of the survey is highlighted in Table 1.
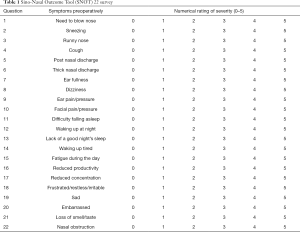
Full table
Overall SNOT 22 scores as well as rhinology-specific sub-domains scores (questions 1, 2, 3, 5, 6, 21 and 22) were analysed. Additional demographic data such as allergies and smoking history were also collected, which enabled for a greater understanding of potential correlations or effect modifiers that may exist between symptomatology and predisposing factors. There did not appear to be any potential confounders and, by using a quantitative scoring tool interview, transfer and recall bias were minimised.
All data for analyses were obtained from an online medical record system present in the hospital which contains scanned copies of patient documents. Patients presenting to the ENT outpatients’ clinic were asked to complete a SNOT 22 survey and a scan of this survey was stored in the patient’s records. During the data collection period, an iPad-based online database system where patients completed a digital SNOT 22 survey to score and upload was also trialled, however, this method of data collection proved logistically problematic and was abandoned during the data collection phase. This trial of iPad data collection only accounted for approximately only 15% of participant data; all data from this collection method was included into the final results. Although this collection method was abandoned all patient scores used through the system were also analysed in the paper. Participants who were unable to use the iPad were automatically offered paper alternative surveys.
Surgical procedure
A vasoconstrictive and local anaesthetic nasal spray (Cophenylcaine) is used to anaesthetise the nasal passage; followed by an injection of the anterior portion of the inferior turbinate with approximately 2 mL of xylocaine/adrenaline. The RFA probe was inserted submucosally, medial to the turbinate bone approximately 10 mm under the mucosa sliding along the turbinate bone. This can be repeated at multiple entry points (usually 2–3) to cover a larger area of the turbinate. One at the very anterior (head) end of the turbinate and a second probe insertion approximately 10 mm posterior to the head of the turbinate will lead to a reduction of turbinate volume. Approximately 10 seconds of energy was delivered per insertion, or until the mucosa starts blanching. Often 2 insertion sites per side were utilised; if the participant had a very large inferior turbinate, a third insertion and ablation was performed. A short observation period of approximately 30 minutes post RFA procedure was recommended. No packing was used. Nasal saline washes were recommended the first week after the RFA procedure. The probes are multi-use with an estimated life span for +500 cycles, and are double the cost of a single use probe.
Statistical analysis
Data were captured in Excel and statistical analysis were performed in SPSS v22.0. An MCID score of 9 points using the SNOT 22 tool has been shown to indicate clinical significance in participants undergoing surgical procedures (28-30). Paired two sample t-tests were used to determine statistical significance.
Results
Of the total number of 189 participants, 98 completed the pre RFA baseline SNOT 22 survey and at least one post-procedure SNOT 22 survey and were included in the SNOT 22 analysis. None of the participants experienced any adverse events as a result of their procedure or were admitted to hospital due to the RFA procedure. A total 97 participants completed a SNOT 22 survey 1-month post RFA and 57 completed it 6 months post RFA. Only 29 participants completed a SNOT 22 survey at all three timepoints. Of the total cohort of 189 participants, only 13 (7%) progressed to surgical treatment despite initial RFA. Notably, 9 (69%) of the 13 patients who progressed to surgery had traumatic and anatomical changes that affected their symptoms and would require surgery to be corrected. Baseline clinical and demographic characteristics of the participants in the sample size are highlighted in Table 2.
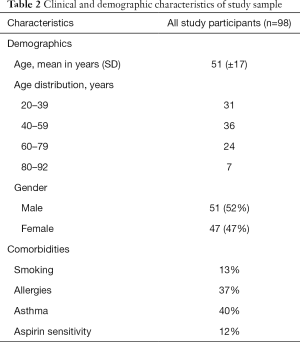
Full table
Of the 98 participants included in the SNOT analysis, 93 (94%) completed the additional demographic information survey which gathered information regarding smoking status, and history of allergies or asthma. Of this sample 13 participants (14%) were smokers, and 37 participants (40%) suffered from asthma. Thirty-four participants (37%) stated they may have allergies and 28 (30%) stated that they have had recurrent infections in childhood. Although these additional characteristics may contribute to effect modification.
Participants’ overall mean SNOT 22 scores for each question and at each time point were used to assess any changes in symptoms. One month after the RFA procedure, there was a statistically significant (P=0.04) improvement in the overall participants’ mean SNOT 22 scores, which correlated to an improvement of 8.0 points, almost reaching minimal clinical importance (MCID 8.9 points). An improvement was also observed at the 6-month follow up, when compared to baseline, these SNOT 22 scores also correlated to an improvement of 8.0 points but this improvement was not statistically significant difference (P=0.19) (Table 3). One month after their RFA procedure, 57% of participants had a 9-point improvement in their SNOT 22 symptom scores from baseline and 6-months after the RFA procedure 46% of participants had improved 9-point in their symptoms from baseline (Figure 1). Overall, a clear clinical improvement in symptoms was observed between baseline and 1-month after the RFA procedure. Of those participants who completed both the baseline and 1-month survey (n=59), 34 (58%) had experienced a MCID. Of the participants who completed both the baseline and the 6-month follow up survey (n=39), 18 (46%) had experienced a MCID.
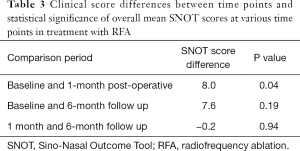
Full table
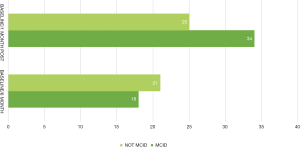
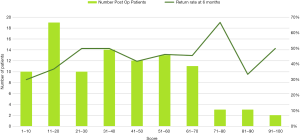
Participants reported a far greater improvement in their QOL symptoms such as sleeping symptoms (Q11, 12, 13, 14), fatigue and productivity (Q16, 17, 18) and most relevant nasal obstruction (Q22). The clinical improvement observed in Q22 (nasal obstruction) was statistically significant at both 1-month post-operative (P=0.018) and 6 months post operatively (P=0.000028). Thirty percent of the 98 participants who filled out a SNOT 22 survey (n=29) completed a survey at all three timepoints. Analysis of this subset of the sample is highlighted in Figure 2 and shows a smaller trend towards improved results, with SNOT 22 scores measured at the 6-month follow up visit indicating a recurrence of symptom severity. An analysis of participant return rates based on their SNOT scores revealed that participants with scores higher than 70 at the 1 month post-operative time frame had a 50% chance of returning at 6 months, when compared to the entire group of post-operative participants who had a return rate of 43%. This has been graphically highlighted in Figure 3.
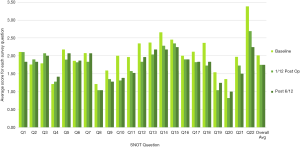
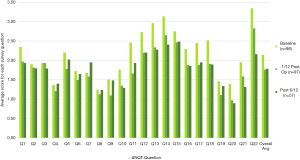
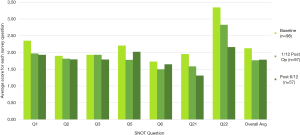
A trend analysis was performed on the total SNOT 22 scores for each question at all three time points and the results were compared to highlight the changes in symptoms over time with reference to each question (Figure 4). A similar analysis was performed on the rhinology specific domains as is highlighted in Figure 5. It must be aforementioned that there is a disparity amongst respondents in each group as indicated in the sample numbers in the legend, with only 57 participants analysed in the 6-month survey. Interestingly the questions that scored changes in sneezing and runny noses (question 2 and 3) did not appear to improve at 1-month post operatively however showed a reduction at 6 months post operatively. How much of this is procedural improvement versus sample size is not adequately discernible.
Economical outcome
The Royal Brisbane & Women’s Hospital New Technology Funding and Evaluation Program calculated the direct cost of RFA of inferior turbinate’s procedure at the ENT outpatient’s clinic to be $184 AUD per patient. The surgical procedure has been estimated at AUD $4,500 to $6,000. In addition to the surgery costs there would be a societal cost of absence from work or reduced working capacity after surgical reduction of inferior turbinate’s, which is generally not necessary after RFA.
Discussion
The data demonstrate that RFA is an effective alternative in the treatment of nasal obstruction from ITH in an Australian hospital setting. From the results gathered, there was no significant difference in the number of males versus females to receive RFA nor was any particular age group more inclined to receive RFA. The historical information provided by participants highlighted that there was a notable number of participants with pre-existing respiratory compromise and allergies, which could predispose them to experiencing the symptoms of ITH more adversely than others. It would be interesting to follow the trajectory of these patients for a longer period of time to assess whether they had improved or poorer symptoms, and to further evaluate the likelihood of these predisposing risk factors contributing to their symptoms.
The impact of RFA on this Australian patient group appears to mirror the results illustrated internationally (31-34). There are statistically significant differences between baseline and 1-month post-RFA patient SNOT scores, which fall in line with the currently evidenced effectivity of RFA (32-34). The presence of this change is however only highlighted between the baseline and 1-month post-operative scores and are not translated in the longer-term observations. This is likely as a consequence of the limited observational data collected at the 6-month timepoint. It is possible that the patients who were doing well were less likely to return to the clinic for what may be considered an obsolete follow up. Another notable outcome of the procedure was the lack of adverse outcomes, as well as the small number of participants that then went on to surgery. Only 13 participants received surgical treatments and this was predominantly (9 patients) patients with traumatic or congenital variations in anatomy. There were only 4 patients proceeding to surgery who experienced recurrence of symptoms without another cause. The trend analysis performed on participants scoring highly 1 month after the procedure appears to corroborate our understanding that they are more likely to present for further follow up reviews, when compared to those with lower SNOT scores.
Basic trend analysis of the SNOT 22 data revealed that all questions other than question two and three, which pertain to sneezing and runny noses, improved immediately after the procedure. This increase in sneezing and runny nose symptoms may be as a result of the acute oedema and crusting that can occur post procedure, and may be something to assess later as this trend persisted in participants who completed all three SNOT 22 surveys (15,32). Interestingly, general QOL symptoms in patients improved markedly in the general patient analysis as well as those patients who only completed all three surveys. This was despite the presence of ongoing symptom complaints such as sneezing or runny nose which appear not to affect these other QOL symptoms. Most importantly the penultimate symptom of nasal obstruction is statistically significant in its improvement pre and 1-month post operatively as well as 6 months post operatively. This emphasises the likely efficacy of the procedure in targeting the most prominent symptomatic complaint in these patients.
Although there was a dropout of participant surveys with each time point, there were a small overlap of participants (n=29) who completed all three surveys. Trends from the SNOT surveys of these participants provides an insight into the potential QOL changes over 6 months. The trends appear to illustrate an acute improvement at one-month post operatively in these participants followed by a slight recession in QOL symptoms at 6 months. Sneezing and runny nose have consistently created symptomatic concerns in these participants and appeared to deteriorate over time. Similarly question five which refers to the post nasal discharge symptoms also progressively worsened at 6 months. It is difficult to ascertain how much of this is attributed to the sample bias of participants returning to the clinic to complete the final 6-month survey.
When accounting for economical costs both direct and indirect, as well as time schedules, waiting lists, lack of complications, and ongoing symptom improvement highlights that RFA used in Australian clinical settings may provide a significantly beneficial outcome. This is a sentiment mirrored by Cavaliere et al. who found that although both surgical treatment and RFA improved patient symptoms significantly, the aforementioned benefits of RFA specifically make it a more clinically applicable and economical treatment option for patients, particularly in public hospitals (16).
The general design of this observational study had some flaws that alter the precision of the findings. A potential sample and attrition bias may have occurred by collecting data from patients who experienced negative outcomes as a result of the procedure. Although all participants who underwent the RFA procedure are made a follow up appointment for 1 month, as well as 6-month follow up appointment. The number of participants returning to discuss and complete a SNOT tool were often likely those experiencing ongoing symptoms particularly at the 6-month follow up. It is likely that those experiencing positive outcomes were less likely to follow up. It would have been ideal to have a dedicated member of the study team immediately call all patients who failed to attend appointments at the 6-month interval to determine their SNOT 22 score. As a result, data obtained from patients willing to attend their 6-month follow up visit may sway towards higher SNOT scores and gives an impression of potentially poorer QOL outcomes.
Conclusions
The use of RFA in the treatment of ITH provides a safe and effective alternative, with low direct and indirect costs compared to septoplasty and surgical reduction of turbinates. Going forward it will be essential to compare the results observed from RFA to the main comparator (septoplasty/reduction of turbinates) and discuss the clinical findings and economical properties of both treatment methods.
Acknowledgments
ENT department at Royal Brisbane and Women’s Hospital, QLD Australia.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-20-52
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ajo-20-52
Peer Review File: Available at http://dx.doi.org/10.21037/ajo-20-52
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-20-52). AC serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Royal Brisbane and Women’s Hospital Human Research Ethics Committee (LNR/2018/QRBW/46297) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jessen M, Malrn L. Definition, prevalence and development of nasal obstruction. Allergy 1997;52:3-6. [Crossref] [PubMed]
- Newson L. Rhinitis and Nasal Obstruction. In: Ear Nose and Troat. 2017. Available online: https://patient.info/doctor/rhinitis-and-nasal-obstruction
- Morcom S, Phillips N, Pastuszek A, et al. Sinusitis. Australian family physician 2016;45:374. [PubMed]
- Health AIo, Welfare. Allergic rhinitis ('hay fever'). Canberra: AIHW2020.
- Bhattacharyya N, Deschler DG, Eamranond P. Clinical presentation, diagnosis, and treatment of nasal obstruction. Retrieved November 2014;12. Available online: https://somepomed.org/articulos/contents/mobipreview.htm?14/59/15280/abstract/30
- Passàli D, Passàli FM, Passàli GC, et al. Treatment of inferior turbinate hypertrophy: a randomized clinical trial. Ann Otol Rhinol Laryngol 2003;112:683-8. [Crossref] [PubMed]
- Stölzel K, Bandelier M, Szczepek AJ, et al. Effects of surgical treatment of hypertrophic turbinates on the nasal obstruction and the quality of life. Am J Otolaryngol 2017;38:668-72. [Crossref] [PubMed]
- Lee KC, Hwang PH, Kingdom TT. Surgical management of inferior turbinate hypertrophy in the office: three mucosal sparing techniques. Oper Tech Otolayngol Head Neck Surg 2001;12:107-11. [Crossref]
- Hol MKS, Huizing EH. Treatment of inferior turbinate pathology: a review and critical evaluation of the different techniques. Rhinology 2000;38:157-66. [PubMed]
- Dawes PJ. The early complications of inferior trubinectomy. J Laryngol Otol 1987;101:1136-9. [Crossref] [PubMed]
- De Corso E, Bastanza G, Di Donfrancesco V, et al. Radiofrequency volumetric inferior turbinate reduction: long-term clinical results. Acta Otorhinolaryngol Ital 2016;36:199. [PubMed]
- Dhulipalla S. Comparative study of response through reduction in the size of hypertrophied inferior turbinate causing nasal obstruction by different surgical modalities: a prospective study. Indian J Otolaryngol Head Neck Surg 2015;67:56-9. [Crossref] [PubMed]
- Li KK, Powell NB, Riley RW, et al. Radiofrequency volumetric tissue reduction for treatment of turbinate hypertrophy: a pilot study. Otolaryngol Head Neck Surg 1998;119:569-73. [Crossref] [PubMed]
- Porter MW, Hales NW, Nease CJ, et al. Long‐term results of inferior turbinate hypertrophy with radiofrequency treatment: a new standard of care? Laryngoscope 2006;116:554-7. [Crossref] [PubMed]
- Sapçı T, Güvenç MG, Evcimik MF. Radiofrequency treatment for inferior turbinate hypertrophy. Kulak Burun Bogaz Ihtis Derg 2011;21:56-60. [PubMed]
- Cavaliere M, Mottola G, Iemma M. Comparison of the effectiveness and safety of radiofrequency turbinoplasty and traditional surgical technique in treatment of inferior turbinate hypertrophy. Otolaryngol Head Neck Surg 2005;133:972-8. [Crossref] [PubMed]
- Nease CJ, Krempl GA. Radiofrequency treatment of turbinate hypertrophy: a randomized, blinded, placebo-controlled clinical trial. Otolaryngol Head Neck Surg 2004;130:291-9. [Crossref] [PubMed]
- Vijay Kumar K, Kumar S, Garg S. A Comparative Study of Radiofrequency Assisted Versus Microdebrider Assisted Turbinoplasty in Cases of Inferior Turbinate Hypertrophy. Indian J Otolaryngol Head Neck Surg 2014;66:35-9. [Crossref] [PubMed]
- Guidance N. Radiofrequency tissue reduction for turbinate hypertrophy. In: Interventional procedures guidance [IPG495]. National Institute for Health and Care Excellence. 2014. Available online: https://www.nice.org.uk/guidance/ipg495/chapter/5-Safety
- Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg 2015;152:S1-S43. [Crossref] [PubMed]
- Holmstrom M. The use of objective measures in selecting patients for septal surgery. Rhinology 2010;48:387-93. [PubMed]
- Adil E, Huntley C, Choudhary A, et al. Congenital nasal obstruction: clinical and radiologic review. Eur J Pediatr 2012;171:641-50. [Crossref] [PubMed]
- Farmer SEJ, Eccles R. Chronic inferior turbinate enlargement and the implications for surgical intervention. Rhinology 2006;44:234. [PubMed]
- Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol 2009;34:447-54. [Crossref] [PubMed]
- Piccirillo JF, Merritt MG Jr, Richards ML. Psychometric and clinimetric validity of the 20-item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg 2002;126:41-7. [Crossref] [PubMed]
- DeConde AS, Mace JC, Bodner T, et al. editors. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. 2014: Wiley Online Library.
- Allen IE, Seaman CA. Likert scales and data analyses. Quality Progress 2007;40:64-5.
- Hopkins C, Rudmik L, Lund VJ. The predictive value of the preoperative Sinonasal outcome test-22 score in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope 2015;125:1779-84. [Crossref] [PubMed]
- Rudmik L, Soler ZM, Mace JC, et al. Using preoperative SNOT-22 score to inform patient decision for endoscopic sinus surgery. Laryngoscope 2015;125:1517-22. [Crossref] [PubMed]
- Steele TO, Rudmik L, Mace JC, et al. editors. Patient-centered decision making: the role of the baseline SNOT-22 in predicting outcomes for medical management of chronic rhinosinusitis. 2016: Wiley Online Library.
- Deenadayal DS, Sudhakshin P, Hameed S. Radiofrequency reduction of inferior turbinates in allergic and non allergic rhinitis. Indian J Otolaryngol Head Neck Surg 2014;66:231-6. [Crossref] [PubMed]
- Hytönen ML, Bäck LJJ, Malmivaara AV, et al. Radiofrequency thermal ablation for patients with nasal symptoms: a systematic review of effectiveness and complications. Eur Arch Otorhinolaryngol 2009;266:1257-66. [Crossref] [PubMed]
- Liu CM, Tan CD, Lee FP, et al. Microdebrider-assisted versus radiofrequency-assisted inferior turbinoplasty. Laryngoscope 2009;119:414-8. [Crossref] [PubMed]
- Sapçi T, Şahin B, Karavus A, et al. Comparison of the effects of radiofrequency tissue ablation, CO2 laser ablation, and partial turbinectomy applications on nasal mucociliary functions. Laryngoscope 2003;113:514-9. [Crossref] [PubMed]
Cite this article as: Chaudhry S, Maresco-Pennisi D, Cervin A. Radiofrequency ablation in the treatment of inferior turbinate hypertrophy. Aust J Otolaryngol 2021;4:5.


