
The association between body size and chronic upper airway disorders
Introduction
Excessive weight and obesity contribute to premature mortality, increased prevalence of chronic disease, greater use of healthcare services, and higher psychological distress and disability (1-3). Overweight and obese individuals are more likely to use medications, visit the emergency department and outpatient clinics, and require hospitalizations (2). Obesity is strongly related to chronic diseases such as cardiovascular disease, chronic kidney disease, diabetes mellitus, back pain, arthritis, and asthma (4).
Obesity has been linked to chronic systemic inflammation by inducing the upregulation of pro-inflammatory adipocytokines and acute phase proteins (5). Chronic upper airway disorders, allergic rhinitis (AR) and chronic rhinosinusitis (CRS), are prevalent sinonasal disorders considered to be perpetuated by significant inflammatory pathways (6,7). AR and CRS are chronic diseases associated with reduced productivity, poor quality of life and frequent use of healthcare services (8,9). Epidemiological studies using population-based health surveys and databases have linked obesity with chronic upper airway disorders (10-12). In the United States, the mean adjusted prevalence of AR and CRS has been shown to rise linearly with increasing body mass index (BMI) (10). In Taiwan, adult obesity was associated with a two-fold increase in the likelihood of CRS in adult residents (13).
In Australia, excessive body weight affects nearly 2 in 3 individuals (14). It is estimated that excessive weight and obesity contribute to 7% of the total healthcare expenditure (4). Although AR and CRS are more common than diabetes mellitus and ischaemic heart disease in Australia (15), sparse discussion has been allocated to the association between body size chronic upper airway disorders. This study’s objective was to determine the association of BMI and waist circumference with symptoms of chronic sinonasal disease in Australia. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-20-75).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (16). As described by the Australian Bureau of Statistics (ABS), data collection was performed in accordance with the Census and Statistics Act 1905. Detailed information of the data collection process, sampling method and other aspects of NHS 2017/18 are published elsewhere (https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/4363.0~2017-18~Main%20Features~Users’%20Guide~1) (17).
This cross-sectional study consisted of a secondary analysis of data collected from the National Health Survey (NHS) 2017/18 conducted by the ABS. The sampling method utilized a stratified, multistage cluster sample of households in all states and territories across urban, rural and remote areas, covering 97% of Australia. Respondents included in this survey provided a nationally representative sample of the Australian population. The valid response rate for the NHS 2017/18 was 76%. The NHS 2017/18 was conducted by the ABS in accordance with the Census and Statistics Act 1905 and National Health and Medical Research Council (17).
Definition of variables
Trained ABS interviewers conducted face-to-face interviews with respondents, focusing on socio-demographic and economic characteristics, lifestyle factors, health status, long-term health conditions, health services use, and medications. A list of the long-term health conditions is described elsewhere (https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/4363.0~2017-18~Main%20Features~Health%20conditions~4) (17). Individuals participating in this study were asked whether they had any long-term conditions that had lasted or were expected to last for 6 months or more via a prompt card. On this card, chronic symptoms of ‘hay fever/allergic rhinitis’ and ‘sinusitis or sinus allergy’ were included as discrete options. Given the focus of NHS 2017/18 on long-term health conditions, ‘hay fever/allergic rhinitis’ was considered symptoms of AR and ‘sinusitis or sinus allergy’ was considered symptoms of CRS, for the purposes of the current study. Methods used in the current study were similar to those from previously published studies using national health surveys in other countries (18,19).
Demographics
Individuals less than 15 years of age were excluded from the analytic sample. For demographic characteristics, age was reported in 5-year increments and re-grouped using the following categories (15–29, 30–44, 45–60, ≥60 years) and gender was classified dichotomously (female, male). Ethnicity was classified according to the Australian Standard Classification of Cultural and Ethnic Groups (20) and grouped into Australian, European, and other (New Zealand Peoples, Melanesian and Papuan, Micronesian, Polynesian, African, Asian, Middle Eastern, People of the Americas).
Cigarette smoking and alcohol consumption
Cigarette smoking status was categorized as an individual-level variable comprised of current, former and never smokers. Current smokers were defined as respondents who smoked daily, weekly or less than weekly. Alcohol consumption was quantified using categories established by the ABS from the National Health and Medical Research Council (NHMRC) for Australia Guidelines for Consumption of Alcohol [2009] (21). Long-term alcohol consumption risk was classified into: exceeding guideline recommendations (average of >2 standard drinks per day), within guideline recommendations (average of ≤2 standard drinks per day) and never consumed alcohol (21).
Body size
A detailed description of the methods used to measure height and weight has previously been described (17). Measurement of height and weight of survey participants was voluntary and carried out by trained interviewers. Height was measured using a stadiometer and recorded in centimeters (cm) correct to two decimal places. Weight was determined using digital platform scales and recorded in kilograms (kg) correct to one decimal point. BMI (kg/m2) was defined as follows: underweight (BMI <18.5), healthy (BMI 18.5 to 24.9), overweight (BMI 25.0 to 29.9), and obese (BMI ≥30.0). Waist circumference was measured using a metal tape measure and recorded in centimeters correct to one decimal point. Interviewers were trained to measure waist circumference using the World Health Organization recommendations (22). Waist circumference groups were defined as follows: females [healthy (<80 cm), increased (≥80 and <88 cm), substantially increased (≥88 cm)] and males [healthy (<94 cm), increased (≥94 and <102 cm), substantially increased (≥102 cm)].
Statistical analysis
A cross-sectional sample of respondents reporting the presence or absence of AR or CRS symptoms were included in the analytic cohort. Sample weights provided by the ABS for the NHS 2017/18 were applied. Odds ratios (OR) and corresponding 95% confidence intervals (95% CI) were calculated to quantify the association between each co-variate and symptomatic AR or CRS. The Chi-squared test was used for univariate testing to assess statistical significance and probability values less than 0.05 were considered statistically significant. A multivariable logistic regression model was constructed to describe the relationship between body size and likelihood of symptomatic AR or CRS, after controlling for age, gender, ethnicity, cigarette smoking and alcohol consumption. All co-variates were entered into the multivariate logistic regression model to evaluate any potential effects of confounding on the relationship between body size and symptoms of chronic sinonasal disease. Statistical analysis was completed using STATA (StataCorp LP, Stata/IC 14.1, USA, 2016).
Results
The survey sample was comprised of 17,248 respondents (age 45.4±19.1 years, 50.1% females) equivalent to a total of 19,501,433 Australians. Most respondents were of Australian ethnicity (67.5%), never smoked (56.5%), and consumed on average ≤2 standard drinks per day (69.3%). The prevalence of respondents reporting AR symptoms was 21.5% and the prevalence of CRS symptoms was 9.8%, (representing 4,202,852 and 1,914,494 Australians respectively). Most participants were overweight or obese (55.8%) and had an increased waist circumference (females ≥80 cm: 65.4%, males ≥94 cm: 58.8%).
AR
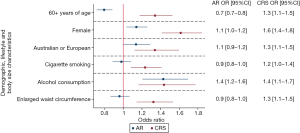
AR symptoms were significantly more likely in respondents ≤60 than >60 years of age [OR 1.2 (1.1–1.4), P<0.001]. The likelihood of AR symptoms did not differ between males and females [OR 1.0 (0.9–1.2), P=0.068]. AR symptoms were more common in respondents of Australian ethnicity than other ethnic counterparts [Australian ethnicity—OR 1.2 (1.1–1.3), P=0.002]. AR symptoms were not associated with smoking status (Table 1). AR symptoms were significantly more likely in respondents who consumed alcohol than those who did not [>2 standard drinks per day—OR 1.4 (1.2–1.7), P<0.001; ≤2 standard drinks per day—OR 1.3 (1.2–1.6), P<0.001].
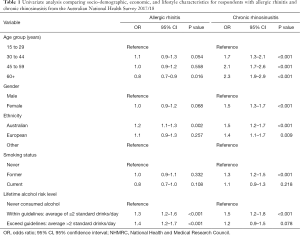
Full table
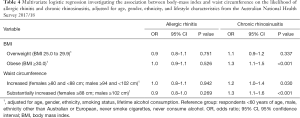
AR symptoms were not associated with BMI (Table 2). AR symptoms were not associated with overweight or obesity compared to counterparts of healthy weight [females—overweight OR 0.9 (0.8–1.1) P=0.705, females—obese OR 1.0 (0.9–1.2) P=0.522; males—overweight OR 0.9 (0.8–1.1), P=0.887, males—obese OR 0.9 (0.7–1.1), P=0.666]. In females, AR symptoms were not associated with waist circumference (Table 2). In males, AR symptoms were significantly less common for respondents with waist circumference ≥102 than <94 cm [OR 0.8 (0.7–0.9), P=0.024].
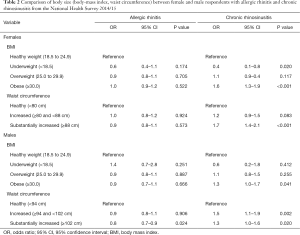
Full table
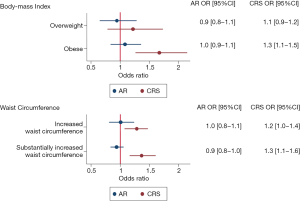
After controlling for age, gender, ethnicity, cigarette smoking and alcohol consumption, AR symptoms were not associated with increased BMI [overweight or obesity—adjusted OR 1.0 (0.9–1.1), P=0.915, Figure 1, Table 3] or enlarged waist circumference [adjusted OR 0.9 (0.8–1.0), P=0.442, Figure 2, Table 3].
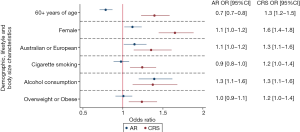
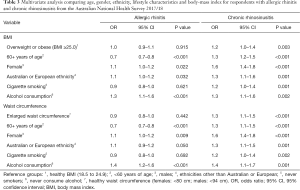
Full table
CRS
CRS symptoms were significantly more common in respondents >60 than ≤60 years of age [OR 2.3 (1.9–2.9), P<0.001]. CRS symptoms were significantly more likely among females than males [OR 1.5 (1.3–1.7), P<0.001]. CRS symptoms were more common in respondents of Australian or European ethnicity than other ethnic counterparts [Australian ethnicity—OR 1.5 (1.2–1.7), P<0.001; European ethnicity—OR 1.4 (1.1–1.7), P=0.009]. Former smokers were significantly more likely to report CRS symptoms than never smokers [OR 1.3 (1.2–1.5), P<0.001]. In univariate analysis, CRS symptoms were not significantly more common in current smokers than never smokers (Table 1). CRS symptoms were significantly more likely in respondents who consumed ≤2 standard drinks per day than those who did not drink alcohol [OR 1.5 (1.2–1.8), P<0.001].
In females, CRS symptoms were significantly more likely in obese than healthy weight respondents [OR 1.6 (1.3–1.9) P<0.001]. CRS symptoms were significantly more common in females with waist circumference ≥88 than <80 cm [OR 1.7 (1.4–2.1), P<0.001]. In males, CRS symptoms were significantly more likely in obese than healthy weight respondents [OR 1.3 (1.0–1.7), P=0.041]. CRS symptoms were significantly more common in males with waist circumference between ≥94 and <102 cm [OR 1.5 (1.1–1.9), P=0.002] and ≥102 cm [OR 1.3 (1.0–1.6), P=0.020] than males with waist circumference <94 cm (Table 2).
After controlling for age, gender, ethnicity, cigarette smoking and alcohol consumption, CRS symptoms were associated with body size [overweight or obese BMI—adjusted OR 1.2 (1.0–1.4), P=0.003, Table 3; increased or substantially increased waist circumference—adjusted OR: 1.3 (1.1–1.5), P<0.001, Table 3]. CRS symptoms were significantly more likely among respondents classified as obese than healthy weight after adjustment [adjusted OR 1.3 (1.1–1.5), P<0.001, Table 4]. Male and female respondents with increased or substantially increased waist circumference were significantly more likely to report CRS symptoms than counterparts with healthy waist circumference after adjustment [increased waist circumference—adjusted OR 1.2 (1.0–1.4), P=0.030; substantially increased waist circumference—adjusted OR 1.3 (1.1–1.6), P<0.001, Figure 3, Table 4].
Full table
Discussion
Obese individuals or individuals with a substantially increased waist circumference (females ≥88 cm; males ≥102 cm) are 1.3 times more likely to report symptoms of CRS than healthy weight counterparts, after controlling for age, gender, ethnicity, cigarette smoking and alcohol consumption.
Obesity is perceived as a hyperinflammatory state with decreased immunologic tolerance, resulting in an increased sensitivity to antigens and subsequent risk of allergy (23). Increased body weight is associated with increased levels of C-reactive protein (CRP), interleukin (IL)-6, IL-8, leptin and TNF-α and decreased levels of adiponectin, contributing to an imbalance between immune activation and regulation (23). In the lower respiratory tract it has been shown that obese individuals with asthma demonstrated increased neutrophilic airway inflammation than non-obese individuals with asthma (24). Circulating levels of CRP, IL-6 and leptin were significantly elevated in obese asthmatics than non-obese asthmatics and non-obese controls (24). Asthma has been shown to co-exist among individuals with chronic upper airway disorders (25,26). In the current study, increased body size was more strongly linked with symptoms of CRS than AR. It is postulated that obesity may lead to a greater neutrophil-mediated airway inflammation common in non-allergic than AR (27). Laboratory studies of obese mice exposed to exhaust fumes exhibit airway inflammation mainly by neutrophils rather than eosinophils, consistent with a largely non-allergic response (28).
The symptom burden experienced by individuals with chronic upper airway disorders may also be associated with gastroesophageal reflux disease (29,30). The symptoms of AR and CRS in this study may not necessarily be sinonasal in nature as they may capture extra-esophageal reflux symptomatology as well. However, it still represents the patient experience as these individuals self-connect with sinonasal disease. Obesity can result in an increase in intragastric pressure and greater frequency of lower esophageal sphincter relaxation or contribute to a hiatal hernia (31). Reflux has been suggested to potentiate chronic upper respiratory airway disorders from direct exposure of nasal and oropharyngeal mucosa to gastric acid, resulting in inflammation and impaired mucocilary clearance (32,33). Alternatively, it is hypothesized that dysfunction of the autonomic nervous system via the vagus nerve may cause reflex sinonasal oedema and obstruction of sinus ostia (32). These mechanisms may lead to downstream sinonasal obstruction, stagnation of mucus, inflammatory stimuli and recurrent infections (32). Despite these hypotheses, the precise pathway by which obesity contributes to sinonasal symptom burden is currently debated (34). Waist loss has been shown to reduce reflux symptoms and may be an additional treatment option to reduce the sinonasal symptom burden for patients with CRS (35).
Measurements of body size (height, weight, waist circumference) utilized for this study were completed using standardized techniques and instruments which were advantageous to lessen the potential for measurement bias. However, this study was limited by multiple factors. Firstly, as a secondary analysis was performed using data collected from a national health survey, this study relied on self-reported AR and CRS status. Although trained interviewers collected data during face-to-face interviews, the nature of self-reporting long-term health conditions relied on patients to report a history of symptoms, diagnosis or an understanding that these conditions should have persisted for 6 months or more. As a result, this method of data acquisition is subject to self-reporting bias and recall bias on the part of the participants. This may overestimate the prevalence of symptomatic AR and CRS in the study sample. Furthermore, the survey questions were pre-determined without specific focus on sinonasal symptoms, treatment, duration of disease status. Although an association was identified between body size and chronic upper airway disorders, the data does not allow consideration of the impact of disease severity.
Despite these limitations, the current study provides a framework to develop future research related to body size and chronic upper airway disorders. Therapeutic strategies promoting weight loss from exercise and dietary modification may be valuable avenues to explore symptom burden improvement and self-esteem. CRS is known to significantly reduce productivity and quality of life (36). It may be important to determine the extent to which body size and perception contributes to quality of life, as these factors may significantly compound the impact of CRS. Overweight or obese patients have been shown to have worse pre-operative nasal endoscopy, total and rhinologic-specific Sinonasal Outcomes Test-22, and total and physical subdomain Rhinosinusitis Disability Index scores compared to healthy weight participants (37). Despite worse pre-operative scores, obese and overweight participants experienced a lesser magnitude of overall improvement in quality of life following endoscopic sinus surgery (ESS) (37). Intraoperatively, obese and overweight individuals required more frequent use of image guidance and more total ethmoidectomies and sphenoidotomies, than counterparts of healthy weight (37). From a clinical standpoint, these findings are important to consider when setting expectations for patients undergoing ESS and surgical planning.
Conclusions
Body size is significantly associated with CRS symptoms. Individuals who are obese or have a substantially increased waist circumference are most likely to report CRS symptoms than healthy weight individuals, after controlling for age, gender, ethnicity, cigarette smoking and alcohol consumption. These findings are relevant given the increasing prevalence of obesity and association with morbidity, mortality and worsening quality of life. Further investigations are warranted to determine the impact of body size on the incidence of chronic upper airway disorders, contribution to sinonasal symptoms, and impact on treatment response.
Acknowledgments
We would like to acknowledge the Australian Bureau of Statistics (ABS) for access to confidential unit files pertaining to the National Health Survey 2017/18 data. Although the research and analysis are based on data collected by the ABS, the opinions expressed do not represent the views of the ABS. This project was presented at the Australian Society of Otolaryngology – Head and Neck Annual Scientific Meeting held from March 22nd to 24th, 2019 in Brisbane, Queensland, Australia.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-20-75
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-20-75). LK and RC serve as unpaid editorial board members of Australian Journal of Otolaryngology from Jan 2019 to Dec 2022. RJH serves as the Editor-in-Chief of Australian Journal of Otolaryngology. LK is on the speaker bureau for Meda Pharmaceuticals, Care Pharmaceuticals and Bayer Pharmaceuticals. RC is on the Speakers bureau and Advisory board for Seqirus. RS is a consultant for Medtronic. RJH is consultant with Novartis, and NeilMed pharmaceuticals. Research grant funding received from Glaxo-Smith-Kline and Stallergenes. He has been on the speakers’ bureau for Seqiris, Astra Zeneca, Meda Pharmaceuticals and Seqirus. He has also been on the speakers’ bureau for Seqirus and MEDA pharmaceuticals. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As described by the Australian Bureau of Statistics (ABS), data collection was performed in accordance with the Census and Statistics Act 1905. Detailed information of the data collection process, sampling method and other aspects of NHS 2017/18 are published elsewhere (https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/4363.0~2017-18~Main%20Features~Users’%20Guide~1).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lung T, Jan S, Tan EJ, et al. Impact of overweight, obesity and severe obesity on life expectancy of Australian adults. Int J Obes (Lond) 2019;43:782-9. [Crossref] [PubMed]
- Reidpath DD, Crawford D, Tilgner L, et al. Relationship between body mass index and the use of healthcare services in Australia. Obes Res 2002;10:526-31. [Crossref] [PubMed]
- Zhou Q, Glasgow NJ, Du W. Health-related lifestyles and obesity among adults with and without disability in Australia: Implication for mental health care. Disabil Health J 2019;12:106-13. [Crossref] [PubMed]
- Australian Institute of Health and Welfare. Impact of overweight and obesity as a risk factor for chronic conditions: Australian Burden of Disease Study. Australian Burden of Disease Study series no.11. Cat. no.BOD 12. BOD. Canberra, Australia, 2017.
- Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006;83:461S-465S. [Crossref] [PubMed]
- Lee S, Lane AP. Chronic Rhinosinusitis as a Multifactorial Inflammatory Disorder. Curr Infect Dis Rep 2011;13:159-68. [Crossref] [PubMed]
- Palmer C, Mulligan JK, Smith SE, et al. The Role of Regulatory T Cells in the Regulation of Upper Airway Inflammation. Am J Rhinol Allergy 2017;31:345-51. [Crossref] [PubMed]
- Chung SD, Hung SH, Lin HC, et al. Health care service utilization among patients with chronic rhinosinusitis: A population-based study. Laryngoscope 2014;124:1285-9. [Crossref] [PubMed]
- Macdonald KI, McNally JD, Massoud E. The health and resource utilization of Canadians with chronic rhinosinusitis. Laryngoscope 2009;119:184-9. [Crossref] [PubMed]
- Bhattacharyya N. Associations between obesity and inflammatory sinonasal disorders. Laryngoscope 2013;123:1840-4. [Crossref] [PubMed]
- Kabeya Y, Kato K, Tomita M, et al. Higher Body Mass Index and Increased Prevalence of Paranasal Sinus Disease. J Epidemiol 2016;26:258-63. [Crossref] [PubMed]
- Han YY, Forno E, Gogna M, et al. Obesity and rhinitis in a nationwide study of children and adults in the United States. J Allergy Clin Immunol 2016;137:1460-5. [Crossref] [PubMed]
- Chung SD, Chen PY, Lin HC, et al. Comorbidity profile of chronic rhinosinusitis: a population-based study. Laryngoscope 2014;124:1536-41. [Crossref] [PubMed]
- Huse O, Hettiarachchi J, Gearon E, et al. Obesity in Australia. Obes Res Clin Pract 2018;12:29-39. [Crossref] [PubMed]
- Habib AR, Campbell R, Kalish L, et al. The burden of chronic upper airway disorders in Australia: a population-based cross-sectional study. Aust J Otolaryngol 2019;2:28. [Crossref]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Australian Bureau of Statistics. National Health Survey: Users’ Guide, 2017-18 2018. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by Subject/4363.0~2017-18~Main Features~Users’ Guide~1
- Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope 2003;113:1199-205. [Crossref] [PubMed]
- Soler ZM, Mace JC, Litvack JR, et al. Chronic rhinosinusitis, race, and ethnicity. Am J Rhinol Allergy 2012;26:110-6. [Crossref] [PubMed]
- Australian Bureau of Statistics. Australian Standard Classification of Cultural and Ethnic Groups 2011. Available online:http://www.abs.gov.au/AUSSTATS/abs@.nsf/allprimarymainfeatures/388FA150E28D57E2CA257FF1001E65F6?opendocument.
- National Health and Medical Research Council. Australian Guidelines to Reduce Health Risks from Drinking Alcohol. Canberra, Australia: Commonwealth of Australia; 2009.
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Geneva, Switzerland, 2008.
- Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy 2007;62:1205-13. [Crossref] [PubMed]
- Scott HA, Gibson PG, Garg ML, et al. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J 2011;38:594-602. [Crossref] [PubMed]
- Habib A-RR, Javer AR, Buxton JA. A population-based study investigating chronic rhinosinusitis and the incidence of asthma. Laryngoscope 2016;126:1296-302. [Crossref] [PubMed]
- Min JYY, Tan BK, Hung SHH, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy 2015;66:1216-23.
- Schleich F, Brusselle G, Louis R, et al. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir Med 2014;108:1723-32. [Crossref] [PubMed]
- Yanagisawa R, Koike E, Ichinose T, et al. Obese mice are resistant to eosinophilic airway inflammation induced by diesel exhaust particles. J Appl Toxicol 2014;34:688-94. [Crossref] [PubMed]
- Leason SR, Barham HP, Oakley G, et al. Association of gastro-oesophageal reflux and chronic rhinosinusitis: systematic review and meta-analysis. Rhinology 2017;55:3-16. [Crossref] [PubMed]
- Sella GCP, Tamashiro E, Anselmo-Lima WT, et al. Relation between chronic rhinosinusitis and gastroesophageal reflux in adults: systematic review. Braz J Otorhinolaryngol 2017;83:356-63. [Crossref] [PubMed]
- Choi CW, Kim GH, Soo SC, et al. Is obesity associated with gastropharyngeal reflux disease? Cheol. World J Gastroenterol 2008;14:265-71. [Crossref] [PubMed]
- Loehrl TA, Smith TL, Darling RJ, et al. Autonomic dysfunction, vasomotor rhinitis, and extraesophageal manifestations of gastroesophageal reflux. Otolaryngol - Head Neck Surg 2002;126:382-7. [Crossref] [PubMed]
- Delehaye E, Dore MP, Bozzo C, et al. Correlation between nasal mucociliary clearance time and gastroesophageal reflux disease: Our experience on 50 patients. Auris Nasus Larynx 2009;36:157-61. [Crossref] [PubMed]
- Kanagalingam S, Shehab SS, Kaminsky DA, et al. Effect of obesity on sinonasal disease in asthma. J Asthma 2018;55:525-31. [Crossref] [PubMed]
- Ness-Jensen E, Lindam A, Lagergren J, et al. Weight Loss and Reduction in Gastroesophageal Reflux. A Prospective Population-Based Cohort Study: The HUNT Study. Am J Gastroenterol 2013;108:376-82. [Crossref] [PubMed]
- Bhattacharyya N. Functional limitations and workdays lost associated with chronic rhinosinusitis and allergic rhinitis. Am J Rhinol Allergy 2012;26:120-2. [Crossref] [PubMed]
- Steele TO, Mace JC, Deconde AS, et al. Does Comorbid Obesity Impact Quality of Life Outcomes in Patients Undergoing Endoscopic Sinus Surgery? Int Forum Allergy Rhinol 2015;5:1085-94. [Crossref] [PubMed]
Cite this article as: Habib AR, Kalish L, Alvarado R, Campbell R, Grayson J, Sacks R, Harvey RJ. The association between body size and chronic upper airway disorders. Aust J Otolaryngol 2021;4:9.


