
Iron deficiency found to be more prevalent in children with adenotonsillar hypertrophy
Introduction
Adenotonsillar hypertrophy is commonly associated with sleep disordered breathing (SDB) with the peak of symptoms between the ages of 3 and 6 (1). Surgery in the form of adenoidectomy with or without tonsillectomy is the current gold standard treatment in this population when their presentation is accompanied by airway obstruction (2). In Australia alone, between 2012–2013, a total of 38,575 tonsillectomies were performed in the paediatric population (3). Whilst the aetiology of adenotonsillar hypertrophy is poorly understood, several hypotheses include allergic disorders, lymphoproliferative disorders, apoptosis, and formation of biofilms with recurrent infections like tonsillitis (4). Nonetheless, none of these are conclusive (4).
SDB encompasses a range of disorders, from primary snoring to severe obstructive sleep apnoea syndrome (5). SDB has significant implications on the paediatric population and has been associated with cardiovascular complications, growth disorders and behavioural problems, such as aggression, hyperactivity, attention-deficit disorder and poor socialisation (6,7). SDB also has implications on neurocognitive functions, such as memory, learning and problem solving ability, are reduced in children with SDB (8). Additionally, Patients with SDB are at increased risk of restless leg syndrome, another paediatric condition associated with iron deficiency (9).
While the role of iron is not definitive in SDB and adenotonsillar hypertrophy, it is known that iron plays an important role in immunity, growth, haematopoiesis and cognitive development (10). Iron deficiency is relatively common with the World Health Organisation estimating a prevalence of 8% of Australian children under the age of 5 and is diagnosed with a serum ferritin level of <20 µg/L (11). This is similar to the United States with a reported prevalence of 7% of children under 5 being iron deficient (9). The presenting symptoms of iron deficiency are varied and widespread. Interestingly, these symptoms are similar to the symptoms children with SDB experience. These include from restless sleep, daytime somnolence and poor attention span (12). In addition, there has been an association between iron deficiency and impaired cognitive function and sleep (13,14). Neurotransmitters affected by iron (dopamine, epinephrine, and serotonin) have been linked to memory, learning, motor control and sleep dysfunction (15). The negative effects of iron deficiency on brain development can be observed later on in life despite having the iron deficiency corrected (16,17).
Furthermore, due to the essential role iron plays in the production of immune cells in the bone marrow, iron deficiency has been associated with dampened function of primary lymphoid organs and thus an increased susceptibility to infection (18). Particularly investigated is the recurrence in paediatric acute respiratory tract infections and gastroenteritis where a correction in iron levels has shown to reduce the rates of these conditions (10). In the pediatric population with coexisting iron deficiency and adenotonsillar hypertrophy, we hypothesise that iron deficiency leading to reduced immunity may result in chronic inflammation and thus adenotonsillar hypertrophy. In specific adult populations, studies suggest iron supplementation reduces the incidence of SDB (4).
It has been postulated that adenotonsillar hypertrophy could result from recurrent infections secondary to poor immune function from iron deficiency. Nonetheless, the current body of literature investigating the relationship between iron deficiency and adenotonsillar hypertrophy is limited. Thus, we have assessed the prevalence of iron deficiency in paediatric populations undergoing adenoidectomy and/or tonsillectomy in order to better understand the role of iron in the etiopathogenesis of adenotonsillar hypertrophy.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-20-89).
Methods
Ethical approval was obtained via institutional Human Research Ethics Committee (reference number 1671) and all patients provided informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was a retrospective point prevalence cohort study across two centres in Western Australia. Participants were recruited between January 2018–September 2019.
The study population was defined as all children 16 years of age or under undergoing adenoidectomy, tonsillectomy or both for either SDB breathing or recurrent tonsillitis. A single surgeon performed all the operations across 2 centres, using the same surgical technique (coblation). A peri-operative blood sample was taken from the intravenous cannula used by the anaesthetist and sent for iron studies (iron, serum ferritin, transferrin and transferrin saturation). If there was sufficient blood from the fresh cannula, a full blood picture (FBP) was also performed.
Data recorded included patient demographics, date and surgical procedure, indication for surgery, iron study results and haemoglobin level. The data was analysed by separate authors to those who performed the operation or collected the data. Key exclusion criteria were based on age; if participants were over the age of 16 at the time of surgery, lack of peri-operative blood sampling; if no iron studies were available or alternative procedure; if the participant did not receive the surgery outlined above.
Participants were followed up 6–8 weeks post operatively as part of routine clinical practice post adenotonsillectomy. No further data was collected for the study during the follow up visit. Participants were made aware of the results of the blood test and if indicated, recommended oral iron supplementation therapy.
Statistical analysis
Statistical analyses were performed using SPSS IBM version 26. A P value of <0.05 was used to indicate a statistically significant result. Iron deficiency was defined as a ferritin level of less than 20 µg/L as per the Royal College of Pathologist Australasia (RCPA) (19). A borderline or equivocal iron level was defined as a ferritin of 20–60 µg/L (19). A low transferrin saturation was defined as less than 15% (20). Anaemia was defined as per the Royal Children’s Hospital criteria based on age and sex, shown in Table 1 (21).
Participants were further divided based on age and diagnosis for subgroup analysis. Age categories were defined as: under 2-year-old, 2 to 6-year-old and over 6-year-old. These cut offs were based on previous literature, as well as physiological and clinical reasons such as growth spurts, surgical patterns and diet (4,11).
Results
In total, 1,712 participants were identified during the recruitment phase; 303 participants were excluded, 9 were above 16-year-old, 198 did not have iron studies performed and 96 did not have an adenoidectomy and/or tonsillectomy. This left a total of 1,409 participants for analysis.
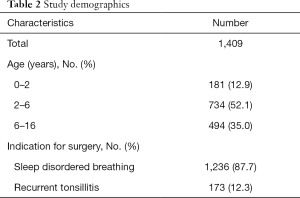
The median age at time of surgery was 4.8 years (standard deviation 3.2, interquartile range 4.2). Further characteristics of the study population are outlined in Table 2.
Full table
478 children were iron deficient (33.9%; 95% CI, 31.3–36.7%) based on a ferritin level of less than 20 µg/L. A further 857 (60.8%) children had a borderline ferritin and of these, 316 (36.9%) had a low transferrin saturation suggestive of iron deficiency that was not identified based on a low ferritin alone (21). Collating these two groups, this study suggests that up to 56.4% of children are iron deficient (Table 3).

Full table
A total of 589 children had a FBP taken. Of these, 142 (24.1%) children were anaemic. 96.5% of children with anaemia had either a low (40.9%) or borderline (55.6%) ferritin level. The remaining 3.5% had ferritin levels of above 60 µg/L (Table 4).

Full table
In a subgroup analysis of iron deficiency, participants with recurrent tonsillitis had a 38.1% prevalence rate of iron deficiency and a 59.0% prevalence rate of borderline ferritin. In the SDB group, the prevalence of iron deficiency and borderline ferritin was 33.3% and 61.2% respectively. There was no statistically significant difference in the prevalence of iron deficiency between the two groups (P=0.245).
Discussion
Our results have shown that the prevalence of iron deficiency in children undergoing surgery for adenotonsillar hypertrophy was significantly higher than the general population. The authors are confident that these results reflect the Australian population given the high number of participants in the study and the narrow 95% confidence interval. In fact, our finding of 33.9% prevalence of low ferritin is likely an underestimate of the total prevalence of iron deficiency in children with adenotonsillar hypertrophy. When evaluating children with a borderline iron deficiency result (ferritin of 20–60 µg/L), the RCPA suggest the use of transferrin saturations should be used to better evaluate iron status in this equivocal group (20). The criteria for borderline ferritin of 20–60 µg/L is a broad range and encompasses the majority of our participants. Part of the reason for the broad range is because ferritin is an acute phase reactant and can be elevated in certain conditions that cause co-existing inflammation, this potentially includes recurrent infections such as tonsillitis or upper respiratory tract infections. Thus, to accurately interpret iron levels in this borderline group, transferrin saturations were used in conjunction. Of the 857 participants with borderline ferritin, 316 (36.9%) had a concurrent low transferrin saturation suggestive of iron deficiency. With these 316 added to the initial 478 with iron deficiency based on low ferritin, our study suggests 56.4% of the study population is iron deficient. To further highlight the importance of the borderline ferritin group, the majority of anaemic patients undergoing surgery had a borderline, rather than a low ferritin level.
More recent studies have suggested that the prevalence of iron deficiency anaemia in Australian children are greater than the 8% quoted by the World Health Organisation. There is a lack of good quality data on this subject (20). The RCPA state that the true prevalence of iron deficiency anaemia in the paediatric population is unknown (19). Ritchie et al reported a prevalence of 39.3% and 30.9% for indigenous and non-indigenous children respectively, who were admitted to hospital (22). The mean age for this group was 15 and 20 months for indigenous and non-indigenous children respectively (22). A similar study in New Zealand found 29% of children aged 9 to 23 months to have iron deficiency anaemia (23). Nevertheless, these reported prevalences are still noticeably lower than the 56.4% suggested by our results.
Age appears to correlate inversely with the frequency of iron deficiency. Our study had the highest rates of iron deficiency in the 0- to 2-year-old group and lowest in the 6- to 16-year-old. This finding replicates those of Kerstein et al., who, in their sample size of 94, also reported higher rates of iron deficiency in ages below 6-year-old and most marked in ages 0 to 2-year-old in children with either restless leg syndrome or periodic limb movement disorder (4). There are additional factors that have effect on iron levels in the less than 2-year-old group. It is well documented that term babies have enough iron stores to meet their requirements for the first four to six months, provided the mother has sufficient intake, but beyond this period the ongoing rapid growth phase and the new need for an iron rich diet explain the high prevalence of iron deficiency (11,12).
While there are multiple theories behind the aetiology of adenotonsillar hypertrophy, none are conclusive (4). The authors subscribe to the theory that adenotonsillar hypertrophy results from recurrent infections secondary to impaired immune function, which could be due to iron deficiency. In our study, no statistically significant difference was found between the rates of iron deficiency between the SDB and recurrent tonsillitis subgroups. This may suggest that iron deficiency plays a role in both conditions although the exact process remains unclear. This finding does not support previous findings of Somuk et al., who reported levels of iron and zinc were significantly lower in the recurrent tonsillitis group (24). However, their study differs in methodology by looking at the amount of iron in tonsillar tissue rather than serologically. Additionally, it was a significantly smaller study with a total of 40 participants. Another trial investigating the effect of adenotonsillectomy on haemoglobin and iron found that there was no difference in serum ferritin before and after the operation, suggesting that low iron may be related to the cause of adenotonsillar hypertrophy, rather than a consequence of it, which supports the study hypothesis (25).
Treatment of iron deficiency in the paediatric population is safe and effective. Due to the many negative consequences of iron deficiency, the National Blood Authority of Australia recommend iron supplementation for all patients with iron deficiency anaemia (11). While there are limited studies that investigate the role of iron in adenotonsillar hypertrophy, in restless leg syndrome, the international restless leg syndrome study group task force recommend treatment with iron supplementation. A Cochrane review on iron treatment for restless leg syndrome found that iron supplementation was better than placebo for reducing severity of symptoms and that side effects were not more common with oral iron compared to placebo (25). The current RCPA treatment guidelines recommend treatment with oral iron supplementation as a first line remedy (19).
Limitations
There are recognised limitations to this study. Firstly, the study design is a cohort prevalence study and although it has a large number of participants, the results can only suggest a correlation between iron deficiency and adenotonsillar hypertrophy rather than causation. Future studies should explore the pathophysiology of adenotonsillar hypertrophy and the effect of iron on this process. An additional research question worth investigating is if iron replacement will benefit the symptoms of SDB or recurrent tonsillitis in this population.
Another limitation is the difference between the number of FBP obtained compared to iron studies. This is due to the issue with drawing sufficient blood for both iron studies and a FBP from the same intravenous cannula. Nevertheless, our study has analysed the haemoglobin from 589 participants.
Our study did not report on ethnicity or gender. Although gender does not play a significant role in prepubescent children, future studies should incorporate this into their data analysis.
Conclusions
In summary, the prevalence of iron deficiency is significantly higher in children with adenotonsillar hypertrophy compared to the general population. The negative consequences of iron deficiency have lasting impacts during development and persist into adulthood, which need to be addressed appropriately. In this population of children with adenotonsillar hypertrophy, iron deficiency appears to be more common in the younger age groups and does not appear to be different between participants with SDB versus recurrent tonsillitis. Future work should be focused on the role of iron in the pathophysiology of adenotonsillar hypertrophy and the benefits of treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-20-89
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ajo-20-89
Peer Review File: Available at http://dx.doi.org/10.21037/ajo-20-89
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-20-89). SV serves as an unpaid editorial board member of the Australian Journal of Otolaryngology from Jan 2019 to Dec 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained via institutional Human Research Ethics Committee (reference number 1671) and all patients provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mitchell RB. Sleep-disordered breathing in children: are we underestimating the problem? Eur Respir J 2005;25:216-7. [Crossref] [PubMed]
- Goldstein NA, Fatima M, Campbell TF, et al. Child behavior and quality of life before and after tonsillectomy and adenoidectomy. Arch Otolaryngol Head Neck Surg 2002;128:770-5. [Crossref] [PubMed]
- Amin RS, Carroll JL, Jeffries JL, et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med 2004;169:950-6. [Crossref] [PubMed]
- Kerstein R, Stimpson P, Caulfield H, et al. Iron deficiency and sleep disordered breathing in children—Cause or effect? Int J Pediatr Otorhinolaryngol 2009;73:275-80. [Crossref] [PubMed]
- Owens J, Spirito A, Marcotte A, et al. Neuropsychological and behavioral correlates of obstructive sleep apnea syndrome in children: a preliminary study. Sleep Breath 2000;4:67-78. [Crossref] [PubMed]
- Probst R, Grevers G, Iro H. Basic otorhinolaryngology: a step-by-step learning guide. Thieme; 2005.
- Greenfeld M, Tauman R, DeRowe A, et al. Obstructive sleep apnea syndrome due to adenotonsillar hypertrophy in infants. Int J Pediatr Otorhinolaryngol 2003;67:1055-60. [Crossref] [PubMed]
- Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci 2014;19:164. [PubMed]
- Munzer T, Felt B. The role of iron in pediatric restless legs syndrome and periodic limb movements in sleep. Semin Neurol 2017;37:439-45. [Crossref] [PubMed]
- Jayaweera JAAS, Reyes M, Joseph A. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci Rep 2019;9:12637. [Crossref] [PubMed]
- National Blood Authority Australia. In: Paediatric and neonatal iron deficiency anaemia guide: Guideline for Australian health providers. Issue ISBN 978-0-9944061-8-7, 2017. Canberra.
- World Health Organisation. In: Iron Deficiency Anaemia. Assessment, Prevention, and Control: A guide for program managers. 2001 World Health Organisation.
- Beard JL. Why iron deficiency is important in infant development. J Nutr 2008;138:2534-6. [Crossref] [PubMed]
- Estrada JA, Contreras I, Pliego-Rivero FB, et al. Molecular mechanisms of cognitive impairment in iron deficiency: alterations in brain-derived neurotrophic factor and insulin-like growth factor expression and function in the central nervous system. Nutr Neurosci 2014;17:193-206. [Crossref] [PubMed]
- Lozoff B, Beard J, Connor J, et al. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 2006;64:S34-S43. [Crossref] [PubMed]
- Lozoff B, Jimenez E, Hagen J, et al. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics 2000;105:E51 [Crossref] [PubMed]
- Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med 2006;160:1108-13. [Crossref] [PubMed]
- Muñoz C, Rios E, Olivos J, et al. Iron, copper and immunocompetence. Br J Nutr 2007;98:S24-8. [Crossref] [PubMed]
- The Royal College of Pathologists of Australasia. Iron studies standardised reporting protocol. RCPA, Sydney; 2013.
- The Royal College of Pathologists of Australasia. The Use of Iron Studies, Ferritin and other tests of iron status. New South Wales; 2017.
- Royal Childrens Hopsital. Clinical Practice Guidelines: Anaemia. Victoria, Australia; 2019 [cited 2019].
- Ritchie B, McNeil Y, Brewster D. Soluble transferrin receptor in Aboriginal children with a high prevalence of iron deficiency and infection. Trop Med Int Health 2004;9:96-105. [Crossref] [PubMed]
- Wilson C, Grant CC, Wall CR. Iron deficiency anaemia and adverse dietary habits in hospitalised children. N Z Med J 1999;112:203-6. [PubMed]
- Somuk BT, Sapmaz E, Soyalıç H, et al. Evaluation of iron and zinc levels in recurrent tonsillitis and tonsillar hypertrophy. Am J Otolaryngol 2016;37:116-9. [Crossref] [PubMed]
- Elverland HH, Aasand G, Miljeteig H, et al. Effects of tonsillectomy and adenoidectomy on hemoglobin and iron metabolism. Int J Pediatr Otorhinolaryngol 2004;68:419-23. [Crossref] [PubMed]
Cite this article as: Weng W, Tan R, Giblett N, Vijayasekaran H, Vijayasekaran S. Iron deficiency found to be more prevalent in children with adenotonsillar hypertrophy. Aust J Otolaryngol 2021;4:21.



