
Laryngeal Botox injection in recalcitrant cases of chronic cough
Introduction
Cough is a physiological protective mechanism which assists in clearance of the airways and prevention of aspiration or inhalation of materials foreign to the lower respiratory tract (1). An acute cough is defined as one lasting less than 3 weeks, while a chronic cough is one which lasts longer than 8 weeks (2). Chronic cough is a common presenting complaint in primary care with a very broad differential list. Although it is a symptom of an underlying disease process, chronic cough itself comes with its own morbidity; including urinary incontinence, anxiety, nausea, fatigue, social isolation and work absenteeism (3). The most common causes are post-nasal drip from allergic rhinitis or chronic sinusitis, cough variant asthma, gastroesophageal reflux disease with laryngopharyngeal reflux, smoking and angiotensin-converting enzyme (ACE) inhibitor medication use (2,4,5). Lung malignancy and obstructive sleep apnoea (OSA) are among other important diagnoses to consider and exclude (6).
There is a growing body of evidence and literature showing a neuropathic model for refractory chronic cough. Going by many names: sensory neuropathic cough, cough hypersensitivity syndrome, irritable larynx syndrome or post viral vagal neuropathy; neurogenic cough is thought to be caused by neural injury from airway inflammatory processes (7). In this model, peripheral sensitisation of the cough reflex at the afferent pathway occurs, with increased cough receptor sensitivity to known cough stimulants (hypertussia), as well as a cough response to normally innocuous stimuli (allotussia). Laryngeal paraesthesia also occurs, where abnormal sensations in the throat causes an inappropriate urge to cough (5,7-12).
When a diagnosis of neurogenic cough is suspected, neurostabiliser medications may be trialled (10,13). Treatment with amitriptyline, gabapentin and pregabalin have all been shown to be beneficial in these cases (13-17). If neurogenic cough is confirmed, speech therapy with respiratory retraining and cough replacement behaviours by a speech-language pathologist is efficacious and should be implemented (18-20). Where these therapies are not successful in treating a neurogenic cough, previous case series have shown that injection of botulinum toxin A (Botox) to the thyroarytenoid muscles as a novel therapy to have good effect (21-23).
Botox is a neurotoxin produced by Clostridium botulinum and its mechanism of action in chronic cough is thought to be two-fold. Firstly, it weakens the adductor muscles by inhibition of presynaptic release of acetylcholine. Secondly, there is evidence for sensory effects, since Botox has been shown to suppress the release of substance P, calcitonin gene-related peptide and glutamate (24), all of which are important neurotransmitters in the cough reflex (1,23,25).
In our specialist laryngology clinic, patients referred with chronic cough are investigated and treated using the standardised ‘Adelaide Protocol’. In this case series study, we took recalcitrant cases of cough where the protocol failed to diagnose and treat the underlying aetiology and treated them using electromyography (EMG) guided Botox injection to the thyroarytenoid muscles.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-21-1).
Methods
This single centre, interventional case series was carried out between 2014 and 2019. The project was approved as a Quality Improvement Project by Southern Adelaide Clinical Human Research Ethics Committee (SAC HREC) and specific ethics review was not sought because the study met criteria for exemption from such review according to an institutional policy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained by each of the participants, who were recruited through our specialist laryngology clinic (Adelaide and Hills ENT, South Australia, Australia), a tertiary referral clinic servicing a population of 1.7 million people. Referrals for investigation and management of chronic cough were accepted from specialists only, including otorhinolaryngology, head and neck surgeons, respiratory physicians, speech and language pathologists, gastroenterologists and allergy & clinical immunologists.
Recruitment to the study required a diagnosis of neurogenic cough, which we made using the Adelaide Protocol to systematically identify and treat causes of chronic cough as follows: a standardised pre-screening questionnaire was filled out by all those referred to our clinic, detailing the cough, outlining previous investigations and treatment, and identifying cough risk factors. All participants required a diagnostic chest X-ray and pulmonary function tests. If an ACE inhibitor was being used, this was discontinued and replaced with an alternative antihypertensive by their general practitioner or cardiologist. If the participant had a diagnosis of asthma, they were managed with appropriate medical therapy as prescribed by their general practitioner or respiratory physician. Where gastroesophageal or laryngopharyngeal reflux was diagnosed, a 6-week trial of high dose proton pump inhibitor, antacid prior to sleep, with dietary and lifestyle risk factor reduction implemented to reduce symptoms. If there was a complete response to this regimen, medications were tapered to the minimal effective dose. If there was a partial response, treatment was escalated by adding barrier agents or prokinetic medications. When a history consistent with allergic rhinitis was elicited, antihistamines and nasal corticosteroids were prescribed empirically. If there was an insufficient response, referral was made to an Immunologist with specific expertise in nasal allergy. Post-nasal drip from chronic rhinosinusitis was treated with standard medical therapy, or surgical intervention where indicated. If screening questions for OSA were positive, polysomnography was performed and appropriate treatment implemented if a diagnosis of OSA was made. Where there was no response to the above treatments, further diagnostic testing was considered, including CT sinuses and modified barium swallow depending on the participants symptoms.
At this point of the Adelaide Protocol, if the participants symptoms persisted, they were deemed to have recalcitrant chronic cough/neurogenic cough. Cough therapy with our speech-language pathologists was implemented, which included education, cough suppression strategies, vocal hygiene training and psychoeducation counselling (26). Neurostabiliser medications were trialled, we used pregabalin (75 mg twice daily) or amitriptyline (up to 25 mg at night). If these interventions for neurogenic cough were not successful after a 6-week trial, or if the neurostabiliser medication was not tolerated, participants proceeded to Botox injection and were recruited to this study.
Exclusion criteria included any reported dysphagia, if this was the case, patients would undergo a swallow assessment prior to consideration of Botox administration. Participants who refused Botox injection were also excluded.
Prior to intervention, participants recruited to the study recorded the frequency and severity of their cough using the following validated questionnaires: cough Visual Analogue Scale (VAS) (27), a self-reported severity scale ranging from zero to ten; Leicester Cough Questionnaire (LCQ), a 19 question quality of life measure of chronic cough with scores ranging from 3 to 21, where a lower score indicates worse cough (28); and the Newcastle Laryngeal Hypersensitivity Questionnaire (NLHQ), a 14 question tool identifying laryngeal sensory disturbance and dysfunction, also scoring between 3 and 21 with lower scores indicative of poorer function (29).
The intervention implemented for those with recalcitrant chronic cough was as follows: a single laryngologist performed all the Botox injections. The approach was a percutaneous midline insertion of a monopolar Teflon coated hollow bore 27-gauge EMG needle attached to a tuberculin syringe, without local anaesthetic, through the cricothyroid membrane, with the needle then angled into the thyroarytenoid muscle bilaterally. On each side, the participant was asked to phonate to augment the EMG signal and ensure correct needle placement prior to injection. One to 2.0 units of Botox was injected to each thyroarytenoid muscle. An average 70 kg person would receive 2.0 units on each side, the dose adjusted to side effects and efficacy.
Participants were followed up 6 weeks after the initial laryngeal Botox injection, or sooner if there were complications. At the 6-week follow up appointment, participants filled out the same questionnaires as pre-procedure (VAS, LCQ, NLHQ), and were screened for complications. Stroboscopic examination of the vocal cords was performed to assess for any subtle paresis not obvious on white light laryngoscopy. Pre- and post-procedure VAS, LCQ and NLHQ scores were compared to assess for change in the participants cough symptoms as a result of the Botox injection.
Treatment efficacy was monitored using the aforementioned questionnaires. Note was made as to whether symptoms recurred at the 3-month post intervention mark, when the therapeutic effect of Botox would have worn off. If symptoms recurred after Botox wore off, reinjection was offered.
Statistical analysis
Statistical analysis was performed with paired t-tests using GraphPad Prism software.
Results
A total of 268 patients with chronic cough were referred to our clinic over the 5-year period between 2014 to 2019. After implementation of the Adelaide Protocol for investigation and treatment of chronic cough, 22 (8.2%) had neurogenic cough unresponsive to neurostabiliser medication and cough therapy. Two patients did not wish to proceed with Botox injection, so 20 of these (7.5%) were recruited for our study and consented for treatment with Botox injection to the thyroarytenoid muscles.
The age of the participants ranged from 28 to 87 years, with a median age of 62.5. There was a female predominance of 17 out of the 20 participants (85%). Average duration of cough at the time of initial consultation was 24.5 months, ranging from 3 to 252 months. One of the 20 participants was an active smoker (5%) and the average daily alcohol intake was 0.95 standard drinks per day (range, 0–6). Asthma was diagnosed and treated in 9 of the 20 (45%) and the average reflux symptom index (30) score of the cohort was 12.5 (range, 1–24) prior to treatment. Occupation was varied, as outlined in Table 1.
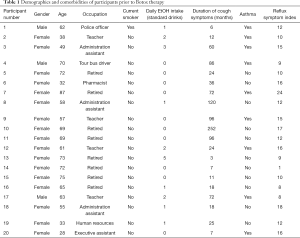
Full table
Six weeks after laryngeal Botox injection, cough severity using the VAS improved from an average score of 7.1 (95% CI: 6.5 to 7.8) to 2.5 (95% CI: 2.0 to 3.0, P<0.001) (Figure 1). LCQ score improved from a mean of 10.8 (95% CI: 9.3 to 12.2) to 14.9 (95% CI: 13.7 to 16.1, P<0.001) (Figure 2). All 20 participants reported improvements in cough using the VAS and LCQ (Table 2). The average NLHQ score improved from 9.1 (95% CI: 8.1 to 10.1) to 14.4 (95% CI: 13.4 to 15.4, P<0.001), with 19 out of 20 (95%) reporting a positively higher score (Figure 3).


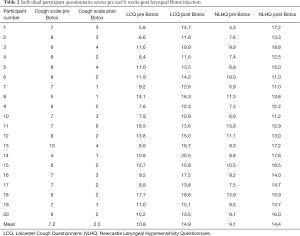
Full table

Treatment was deemed to be successful when cough symptoms had resolved after the Botox wore off 3 months post injection. Treatment success was achieved in 14 out of 20 participants (70%) after a single treatment with Botox injection, 12 of these followed up to 12 months and 2 participants followed up to 6 months with resolution of cough symptoms. One participant (5%) required a second injection to achieve treatment success, where symptoms recurred after the Botox wore off following the first treatment, but repeat injection provided sustained symptom resolution. Five participants (25%) underwent repeat Botox injection (more than four separate injections) without treatment success, where cough symptoms recurred after the Botox wore off each time.
Complications of the procedure included dysphonia and dysphagia. Dysphonia was reported in 3/20 (15%) of participants, 1 with a moderately weak voice and 2 with mild weakness. Minor dysphagia was common in the first 2 weeks after Botox injection, seen in 5 participants (25%), and anecdotally correlated with improvement in cough.
Discussion
Our results demonstrate positive outcomes, with significant improvement of chronic cough symptoms after laryngeal Botox injection. The patient cohort selected for therapeutic Botox injection included those who failed to respond to the Adelaide Protocol of sequential assessment and multi-faceted treatment for chronic cough. As outlined in our results, only a minority of patients actually had recalcitrant cough after receiving care consistent with the protocol (8.2%). For those who have ongoing cough despite the Adelaide Protocol, Botox is a promising therapeutic option to alleviate symptoms and therefore improve quality of life.
These results support the current published literature where Botox has been used successfully as a therapeutic option for chronic cough. Chu et al. (21) published a case series of 4 patients with chronic cough who underwent recurrent Botox injections until cough resolved, with duration of treatment lasting between 7 and 43 months. Sipp et al. (22) used laryngeal Botox for habitual cough in three children, with short-term resolution of symptoms in all patients. Sasieta et al. (23) demonstrated patient reported improvement in cough severity in half of the 22 patients who underwent thyroarytenoid Botox injection for refractory chronic cough.
A notable observation we made of the patients who proceeded to Botox intervention, were the occupations of our patients. Of the patients who were not retired, their occupations were ones where voice use at work was essential (teacher, administration work, police officer, tour bus guide, executive assistant—see Table 1). Our hypothesis is that these patients are unable to break the cough-inflammation cycle involved in their chronic cough, due to work commitments which do not allow voice rest. Therefore, the requirement of their occupation to continuously use their voice may be a factor contributing to the persistent nature of their cough.
Speech therapy is a key factor in treatment of chronic cough, using respiratory retraining therapy and cough replacement behavioural modification (18-20). We believe concurrent input from Speech-Language Pathology in the treatment of chronic cough to be essential for successful treatment outcomes. Early implantation of speech therapy when neurogenic cough is suspected forms part of our Adelaide Protocol, with input continuing throughout treatment with laryngeal Botox.
We found the most common side effects of mild dysphonia and dysphagia to be consistent with previous studies (21-23). Dysphonia, which in our cohort was reported as a degree of “breathiness” in the voice, is likely to accompany treatment success. This is due to the desired treatment effect of weakening laryngeal adductor muscles which also results in decreased ability to achieve glottic apposition during phonation. Dysphagia has previously been postulated to be the result of Botox diffusion into the pharyngeal area (23). In our clinic we use pre-existing dysphagia as a relative contraindication to Botox treatment, where any underlying dysphagia must be investigated and treated prior to consideration of laryngeal Botox therapy. Given the predictable nature of the side effects, we counsel our patients that a mild degree of dysphagia and dysphonia is expected after treatment.
A strength of our study includes the structured approach of the Adelaide Protocol in patient selection for Botox therapy, as this is able to accurately identify those with neurogenic chronic cough. Other strengths include all injections being performed by a single laryngologist, to avoid user variability and reduce error, as well as using multiple validated patient outcome questionnaires to accurately report symptom severity, quality of life and laryngeal sensory function. A limitation of the study is the absence of any objective measure of cough severity, which is consistent with previous reported studies (21-23). In addition, the relatively short duration of follow-up means there is no long-term outcome data on long-term success and symptom recurrence rates.
Future directions for research include randomised control trials using laryngeal injections with Botox vs. placebo, as well as Botox alone vs. Botox plus concurrent therapy with neuromodulator medications. Studies with larger patient cohorts as well as a longer duration of follow up are needed to better characterise the efficacy and long-term effects and relapse rates of laryngeal Botox therapy.
Conclusions
We have found the therapeutic use of laryngeal Botox injection to the thyroarytenoid muscles to be a successful option in recalcitrant cases of chronic cough, with 70% of our patients reporting resolution of cough symptoms.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-21-1
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ajo-21-1
Peer Review File: Available at http://dx.doi.org/10.21037/ajo-21-1
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-21-1). TA serves as an unpaid editorial board member of the Australian Journal of Otolaryngology from Jan 2019 to Dec 2022. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The project was approved as a Quality Improvement Project by Southern Adelaide Clinical Human Research Ethics Committee (SAC HREC) and specific ethics review was not sought because the study met criteria for exemption from such review according to an institutional policy. Written informed consent was obtained by each of the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Polverino M, Polverino F, Fasolino M, et al. Anatomy and neuro-pathophysiology of the cough reflex arc. Multidiscip Respir Med 2012;7:5. [Crossref] [PubMed]
- Morice AH, Kastelik JA. Cough. 1: Chronic cough in adults. Thorax 2003;58:901-7. [Crossref] [PubMed]
- French CL, Irwin RS, Curley FJ, et al. Impact of chronic cough on quality of life. Arch Intern Med 1998;158:1657-61. [Crossref] [PubMed]
- Natt RS, Earis JE, Swift AC. Chronic cough: a multidisciplinary approach. J Laryngol Otol 2012;126:441-4. [Crossref] [PubMed]
- Morrison RJ, Schindler JS. Evaluation and treatment of the patient with chronic cough referred to the otolaryngologist. Laryngoscope 2011;121:S256. [Crossref]
- Sundar KM, Daly SE. Chronic cough and OSA: a new association? J Clin Sleep Med 2011;7:669-77. [Crossref] [PubMed]
- Chung KF, McGarvey L, Mazzone SB. Chronic cough as a neuropathic disorder. Lancet Respir Med 2013;1:414-22. [Crossref] [PubMed]
- Vertigan AE, Gibson PG. Chronic refractory cough as a sensory neuropathy: evidence from a reinterpretation of cough triggers. J Voice 2011;25:596-601. [Crossref] [PubMed]
- Bucca CB, Bugiani M, Culla B, et al. Chronic cough and irritable larynx. J Allergy Clin Immunol 2011;127:412-9. [Crossref] [PubMed]
- Greene SM, Simpson CB. Evidence for sensory neuropathy and pharmacologic management. Otolaryngol Clin North Am 2010;43:67-72. viii. [Crossref] [PubMed]
- Amin MR, Koufman JA. Vagal neuropathy after upper respiratory infection: a viral etiology? Am J Otolaryngol 2001;22:251-6. [Crossref] [PubMed]
- Rees CJ, Henderson AH, Belafsky PC. Postviral vagal neuropathy. Ann Otol Rhinol Laryngol 2009;118:247-52. [Crossref] [PubMed]
- Cohen SM, Misono S. Use of specific neuromodulators in the treatment of chronic, idiopathic cough: a systematic review. Otolaryngol Head Neck Surg 2013;148:374-82. [Crossref] [PubMed]
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:1583-9. [Crossref] [PubMed]
- Halum SL, Sycamore DL, McRae BR. A new treatment option for laryngeal sensory neuropathy. Laryngoscope 2009;119:1844-7. [Crossref] [PubMed]
- Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol 2005;114:253-7. [Crossref] [PubMed]
- Jeyakumar A, Brickman TM, Haben M. Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neuropathy. Laryngoscope 2006;116:2108-12. [Crossref] [PubMed]
- Bonilha HS, Gerlach TT, Sutton LE, et al. Laryngeal sensation before and after clearing behaviors. J Voice 2012;26:674.e1-7. [Crossref] [PubMed]
- Murry T, Tabaee A, Aviv JE. Respiratory retraining of refractory cough and laryngopharyngeal reflux in patients with paradoxical vocal fold movement disorder. Laryngoscope 2004;114:1341-5. [Crossref] [PubMed]
- Murry T, Branski RC, Yu K, et al. Laryngeal sensory deficits in patients with chronic cough and paradoxical vocal fold movement disorder. Laryngoscope 2010;120:1576-81. [Crossref] [PubMed]
- Chu MW, Lieser JD, Sinacori JT. Use of botulinum toxin type A for chronic cough: a neuropathic model. Arch Otolaryngol Head Neck Surg 2010;136:447-52. [Crossref] [PubMed]
- Sipp JA, Haver KE, Masek BJ, et al. Botulinum toxin A: a novel adjunct treatment for debilitating habit cough in children. Ear Nose Throat J 2007;86:570-2. [Crossref] [PubMed]
- Sasieta HC, Iyer VN, Orbelo DM, et al. Bilateral thyroarytenoid botulinum toxin type A injection for the treatment of refractory chronic cough. JAMA Otolaryngol Head Neck Surg 2016;142:881-8. [Crossref] [PubMed]
- Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol 2005;53:3-9. [Crossref] [PubMed]
- Canning BJ, Chang AB, Bolser DC, et al. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest 2014;146:1633-48. [Crossref] [PubMed]
- Ryan NM, Vertigan AE, Bone S, et al. Cough reflex sensitivity improves with speech language pathology management of refractory chronic cough. Cough 2010;6:5. [Crossref] [PubMed]
- Chang AB, Newman RG, Carlin JB, et al. Subjective scoring of cough in children: parent-completed vs child-completed diary cards vs an objective method. Eur Respir J 1998;11:462-6. [Crossref] [PubMed]
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [Crossref] [PubMed]
- Vertigan AE, Bone SL, Gibson PG. Development and validation of the Newcastle laryngeal hypersensitivity questionnaire. Cough 2014;10:1. [Crossref] [PubMed]
- Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice 2002;16:274-7. [Crossref] [PubMed]
Cite this article as: Cook L, Athanasiadis T. Laryngeal Botox injection in recalcitrant cases of chronic cough. Aust J Otolaryngol 2021;4:24.


