
The effect of chlorhexidine mouthwash on bacterial microcolonies in recurrent tonsillitis
Introduction
Chlorhexidine is a broad-spectrum antiseptic that targets microbial biofilms. Mouthwashes containing the active ingredient chlorhexidine digluconate were introduced in the 1970’s and have been extensively marketed since (1-3). Chlorhexidine mouthwash is most commonly used as an adjunct to mechanical oral hygiene procedures to reduce plaque levels and improve gingival health (1). There is high-quality evidence that chlorhexidine mouthwash results in a reduction in dental plaque and has a mild effect in the reduction of gingivitis (1,4).
Chlorhexidine is effective against both Gram-positive and Gram-negative bacteria as well as some viruses and fungi (2). It is cationic and works by forming a strong bond with anionic sites of the cell wall and membrane of pathogens (2,5). This bonding event affects the metabolic capability and osmoregulatory of the pathogen’s cell membrane and enzymes contained within (2,6). If the pathogen is exposed to a high concentration of chlorhexidine, it will result in a loss of structural membrane integrity and subsequent leakage of cellular material (2).
Recurrent acute tonsillitis (RT) is associated with (7) multiple pathogenic bacteria, including Streptococcus sp, Haemophilus influenzae, Staphylococcus aureus, and Actinomyces (7). RT is one of the most common diseases affecting children worldwide (8). Treatment of RT usually includes multiple courses of oral antibiotics and surgical removal (tonsillectomy) is often required to alleviate recurrent symptoms (8). It is likely that the extensive crypt system that exists in the palatine tonsils harbor pathogenic bacterial microcolonies that result in disease (9). Chlorhexidine mouthwash is reported to have immediate antibacterial effects so potent that it takes the oral bacterial population more than three hours to return to baseline bacterial vitality levels following a single mouthwash (2). We hypothesized that chlorhexidine mouthwash may be an alternative to oral antibiotics in eradicating the pathogenic bacteria that cause RT. This study aimed to determine the effects of chlorhexidine mouthwash on the bacterial microcolonies of ex-vivo palatine tonsils from children with RT. A second group of RT patients whose left tonsils were exposed to 0.9% sodium chloride (normal saline) after surgery were included to control for the potential effect of mechanical washing on the microcolonies.
Methods
Participant information
Twenty-one participants undergoing tonsillectomy were recruited for this study. Participants were under the age of 16 years and had taken no antibiotics for at least eight weeks before surgery. All of the participants were having surgery for the indication of RT as per the Paradise criteria (10). This criterion has defined clinically significant RT (requiring tonsillectomy) as seven episodes in one year, five events per year for two years, or three or more episodes per year for three years (10,11).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was obtained from New Zealand Health and Disability Ethics Committee (17/NTB/76) and all patients provided informed consent.
The following demographic data were collected: age, sex, BMI, and ethnicity. Clinical data included the tonsil grade (12), medical comorbidities, antibiotics prescribed in the community and throat swab for culture before admission.
Statistical analysis
Participant demographics and clinical characteristics were summarised using descriptive statistics. Univariate analysis was used to assess any demographic or clinical differences that may exist between participants from the chlorhexidine group versus the saline group. Chi-square tests were performed to assess categorical variables and the Student’s t-test was performed to assess continuous variables. A two-tailed P-value of less than 0.05 was regarded as statistically significant. All statistical analyses were performed by IBM® SPSS® version 24 software.
Sample collection
Tonsillectomy was performed under general anaesthetic by a paediatric ORL surgeon. Intravenous antibiotics were not administered at induction. Following induction, extracapsular tonsillectomy was performed with either bipolar diathermy or coblation diathermy (Smith & Nephew, London, United Kingdom). Each tonsil was removed and placed immediately into individual sterile pots. These samples were stored on ice and taken to the laboratory for processing within one hour of collection.
The left tonsil from each participant in the chlorhexidine group was submerged in 0.2% chlorhexidine digluconate mouthwash under sterile conditions. The tonsil was gently oscillated for 30 seconds and then fixed in formalin. The left tonsils from all participants in the saline group were submerged in a sterile 0.9% sodium chloride solution. The tonsil was also gently oscillated for 30 seconds and fixed in formalin. The right tonsils from all participants in both groups were transferred from the sterile collection pots and fixed directly in formalin.
Histological analysis
Following relevant exposure, the right and left palatine tonsils from all participants were fixed in formalin then embedded in paraffin wax. Tonsils from the participant’s left-hand side had been exposed to either chlorhexidine mouthwash or saline before fixation. Tonsils from the right-hand side were fixed immediately following tonsillectomy and were used as controls. Each palatine tonsil was sectioned in the coronal plane at 250 µm intervals, with 4 µm thick sections being cut at each point. This resulted in approximately 25 sections per tonsil. A Gram stain was performed on each coronal section of the 42 tonsils, followed by a counterstain with safranin to identify Gram-negative bacteria. All sections were screened at ×40 magnification on a Leica DMR upright microscope looking for the presence or absence of bacterial microcolonies in each section. A bacterial microcolony was defined as a colony of bacteria visible only under a low power microscope (×40 magnification).
Results
Participant information
The demographics and clinical characteristics of the 21 participants from this study are shown in Table 1. There were no statistically significant demographic or clinical differences between the two groups of participants. Medications prescribed in the year preceding surgery for each participant in this study were obtained by accessing community prescribing data, which are available to clinicians nationwide in New Zealand. Mean number of courses of antibiotics prescribed to participants in the year before surgery was 3.8±0.8 (range, 2–5) in the chlorhexidine group and 4.0±0.8 (range, 2–6) (P=0.72) in the saline group. A general practitioner had performed a throat swab for detection of Group A Streptococci (GAS) in all participants prior to surgery. These swab results were positive for GAS in all participants at least once in the year before surgery.

Full table
Histological analyses
All available histological slides were reviewed for the presence of bacterial microcolonies (Figure 1A,B). There were significantly fewer bacterial microcolonies observed in the tonsils that were exposed to chlorhexidine mouthwash when compared with the saline control group. The mean number of microcolonies in the saline group was 67±43 (range, 3–160) versus the mean number in the chlorhexidine group = 11±6 (range, 0–27) (P<0.05). When the right tonsils (unexposed) from participants in the saline group were compared with those from the chlorhexidine group, no difference (P=0.68) was observed in the mean number of bacterial microcolonies (saline group = 64±32 (range, 3–122), chlorhexidine group = 57±28 (range, 6–135).
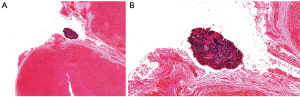
The right and left tonsils from participants in the saline group were compared with one another. No difference was observed in the mean number of bacterial microcolonies (left (exposed) tonsil = 68±43 (range, 3–164), right (unexposed) tonsil = 64±32 (range, 3–122) (P=0.89). Finally, the right and left tonsils from participants in the chlorhexidine group were compared with one another. A significant difference was observed in the mean number of bacterial microcolonies (left (exposed) tonsil = 11±6 (range, 0–27), right (unexposed) tonsil = 57±28 (range, 6–135) (P<0.05).
Discussion
Chlorhexidine mouthwash appears to be effective in significantly reducing the number of bacterial microcolonies that exist in the crypts of these participants with RT. The reduction of bacterial microcolonies in the crypts of patients with RT is essential, as these likely result in the exudative nature of tonsils in disease (13-16), leading to symptoms including halitosis, odynophagia and fever (9).
It is possible that these bacterial microcolonies seen in the tonsils of patients with RT represent the initial step in the formation of tonsilloliths. Several studies have analyzed the composition of clinically evident tonsilloliths (17-24). One group who investigated a tonsillolith with Gram stain found on the surface of the tonsillolith Gram-positive cocci and Gram-positive bacilli. Gram-negative bacilli were observed deep inside the tonsillolith (18). This is consistent with our observations of bacterial micro-colonies (7). Another group which analyzed tonsilloliths microscopically demonstrated a mass of Gram-positive, Gomori methenamine silver positive, periodic-acid Schiff positive, and non-acid fast branched filamentous bacteria (19). If bacterial microcolonies represent early tonsilloliths, then eradication with chlorhexidine mouthwash may prevent tonsillolith formation.
Although the mechanism of action is not entirely clear, the fact that chlorhexidine mouthwash significantly reduces the number of bacterial microcolonies is a potentially clinically significant finding. Oral antibiotics may also be useful in the reduction of the bacterial burden in tonsillitis (25-28). However, unlike chlorhexidine mouthwash, oral antibiotics have many side effects and may detrimentally alter the gut microbiome for a significant period (29,30). Multiple courses of antibiotics may lead to increasing bacterial resistance (31,32). Chlorhexidine mouthwash is a topical treatment that is not swallowed and therefore has only a limited effect on the body's microbiome. It is inexpensive and does not require a prescription.
It should be noted that rinsing with chlorhexidine mouthwash for longer than four weeks can cause adverse effects including calculus formation, extrinsic tooth staining and transient taste disturbance (1). Therefore, the use of chlorhexidine mouthwash should only be recommended when a patient has symptoms of RT or as a preventative measure to be used for one week in every six weeks. A major limitation is that chlorhexidine mouthwash can only be used in patients old enough to gargle mouthwash for at least 30 seconds.
There are several limitations to this study. The cohort of patients is small, and although the findings of this study demonstrate significant differences between groups, the small number of patients must be considered when interpreting these data. The effect of chlorhexidine was determined in vitro after the tonsils had been removed and effects may not be the same in vivo.
Conclusions
Chlorhexidine mouthwash reduces foci of pathogenic bacteria in the crypts of tonsils that have been found to contribute to the chronicity and recurrence of disease in RT (13,14,16). This effect was achieved with a single in vitro exposure. This same effect was not seen in tonsils that were exposed to normal saline. Further studies are required to determine the method of action, duration of response and effect on patient symptoms in vivo. Chlorhexidine mouthwash may offer a non-invasive, topical alternative to treatment in patients with RT.
Acknowledgments
We would like to acknowledge the Garnett Passe and Rodney Williams Memorial Foundation. Without their support, this research would not be possible.
Funding: This work was supported by the Garnett Passe and Rodney Williams Memorial Foundation Surgeon Scientist Scholarship awarded to James Johnston for work towards a Doctor of Philosophy at the University of Auckland.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ajo-19-58
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-19-58). The authors report that this work was supported by the Garnett Passe and Rodney Williams Memorial Foundation Surgeon Scientist Scholarship awarded to James Johnston for work towards a Doctor of Philosophy at the University of Auckland. RGD served as an unpaid editorial board member of the Australian Journal of Otolaryngology from Jan 2019 to Dec 2020.The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was obtained from New Zealand Health and Disability Ethics Committee (17/NTB/76) and all patients provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Richards D. Chlorhexidine mouthwash plaque levels and gingival health. Evid Based Dent 2017;18:37-8. [Crossref] [PubMed]
- Quintas V, Prada-López I, Donos N, et al. In situ neutralisation of the antibacterial effect of 0.2% Chlorhexidine on salivary microbiota: Quantification of substantivity. Arch Oral Biol 2015;60:1109-16. [Crossref] [PubMed]
- Löe H, Schiøtt CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res 1970;5:79-83. [Crossref] [PubMed]
- da Costa LFNP, Amaral CDSF, Barbirato DDS, et al. Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: A meta-analysis. J Am Dent Assoc 2017;148:308-18. [Crossref] [PubMed]
- Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol 2005;99:703-15. [Crossref] [PubMed]
- Hugo WB, Longworth AR. The effect of chlorhexidine on the electrophoretic mobility, cytoplasmic constituents, dehydrogenase activity and cell walls of Escherichia coli and Staphylococcus aureus. J Pharm Pharmacol 1966;18:569-78. [Crossref] [PubMed]
- Johnston JJ, Douglas R. Adenotonsillar microbiome: an update. Postgrad Med J 2018;94:398. [Crossref] [PubMed]
- Johnston J, McLaren H, Mahadevan M, et al. Clinical characteristics of obstructive sleep apnea versus infectious adenotonsillar hyperplasia in children. Int J Pediatr Otorhinolaryngol 2019;116:177-80. [Crossref] [PubMed]
- Johnston J, Hoggard M, Biswas K, et al. The bacterial community and local lymphocyte response are markedly different in patients with recurrent tonsillitis compared to obstructive sleep apnoea. Int J Pediatr Otorhinolaryngol 2018;113:281-8. [Crossref] [PubMed]
- Paradise JL. Tonsillectomy and adenoidectomy. Pediatr Clin North Am 1981;28:881-92. [Crossref] [PubMed]
- Munck H, Jørgensen AW, Klug TE. Antibiotics for recurrent acute pharyngo-tonsillitis: systematic review. Eur J Clin Microbiol Infect Dis 2018;37:1221-30. [Crossref] [PubMed]
- Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am 1989;36:1551-69. [Crossref] [PubMed]
- Chole RA, Faddis BT. Anatomical Evidence of Microbial Biofilms in Tonsillar Tissues: A Possible Mechanism to Explain Chronicity. Arch Otolaryngol Head Neck Surg 2003;129:634-6. [Crossref] [PubMed]
- Al-Mazrou KA, Al-Khattaf AS. Adherent Biofilms in Adenotonsillar Diseases in Children. Arch Otolaryngol Head Neck Surg 2008;134:20-3. [Crossref] [PubMed]
- Galli J, Calò L, Ardito F, et al. Biofilm formation by Haemophilus influenzae isolated from adeno-tonsil tissue samples, and its role in recurrent adenotonsillitis. Acta Otorhinolaryngol Ital 2007;27:134-8. [PubMed]
- Drago L, De Vecchi E, Torretta S, et al. Biofilm formation by bacteria isolated from upper respiratory tract before and after adenotonsillectomy. APMIS 2012;120:410-6. [Crossref] [PubMed]
- Sakano H, Thaker AI, Davis GE. Adenoid Stones - "Adenoliths J Otol Rhinol 2015;4:240. [PubMed]
- Yellamma Bai K, Vinod Kumar B. Tonsillolith: A polymicrobial biofilm. Med J Armed Forces India 2015;71:S95-8. [Crossref] [PubMed]
- Cohen PR, Tschen JA. Tonsillar actinomycosis mimicking a tonsillolith: colonization of the palantine tonsil presenting as a foul‐smelling, removable, unilateral, giant tonsillar concretion. Int J Dermatol 2010;49:1165-8. [Crossref] [PubMed]
- Stoodley P, deBeer D, Longwell M, et al. Tonsillolith: Not just a stone but a living biofilm. Otolaryngol Head Neck Surg 2009;141:316-21. [Crossref] [PubMed]
- Tsuneishi M, Yamamoto T, Kokeguchi S, et al. Composition of the bacterial flora in tonsilloliths. Microbes Infect 2006;8:2384-9. [Crossref] [PubMed]
- Mesolella M, Cimmino M, Di Martino M, et al. Tonsillolith. Case report and review of the literature. Acta Otorhinolaryngol Ital 2004;24:302-7. [PubMed]
- Kodaka T, Debari K, Sano T, et al. Scanning electron microscopy and energy-dispersive X-ray microanalysis studies of several human calculi containing calcium phosphate crystals. Scanning Microsc 1994;8:241-56. [PubMed]
- Dale JW, Wing G. Clinical and technical examination of a tonsillolith: a case report. Aust Dent J 1974;19:84-7. [Crossref] [PubMed]
- Chen LE, Shen YZ, Jiang DY, et al. Amoxicillin and clavulanate potassium in treating children with suppurative tonsillitis. J Biol Regul Homeost Agents 2017;31:625-9. [PubMed]
- El Hennawi DED, Geneid A, Zaher S, et al. Management of recurrent tonsillitis in children. Am J Otolaryngol 2017;38:371-4. [Crossref] [PubMed]
- Windfuhr JP, Toepfner N, Steffen G, et al. Clinical practice guideline: tonsillitis I. Diagnostics and nonsurgical management. Eur Arch Otorhinolaryngol 2016;273:973-87. [Crossref] [PubMed]
- Shetty A, Mills C, Eggleton K. Primary care management of group A streptococcal pharyngitis in Northland. J Prim Health Care 2014;6:189-94. [Crossref] [PubMed]
- Juul ML, Rasmussen ER, Rasmussen SHR, et al. A nationwide registry-based cohort study of incidence of tonsillectomy in Denmark, 1991-2012. Clin Otolaryngol 2018;43:274-84. [Crossref] [PubMed]
- Erickson BK, Larson DR, Sauver JLS, et al. Changes in incidence and indications of tonsillectomy and adenotonsillectomy, 1970-2005. Otolaryngol Head Neck Surg 2009;140:894-901. [Crossref] [PubMed]
- Guss JD, Horsfield MW, Fontenele FF, et al. Alterations to the Gut Microbiome Impair Bone Strength and Tissue Material Properties. J Bone Miner Res 2017;32:1343-53. [Crossref] [PubMed]
- Lima-Ojeda JM, Rupprecht R, Baghai TC. “I Am I and My Bacterial Circumstances”: Linking Gut Microbiome, Neurodevelopment, and Depression. Front Psychiatry 2017;8:153. [Crossref] [PubMed]
Cite this article as: Johnston J, Biswas K, Waldvogel-Thurlow S, Radcliff FJ, Mahadevan M, Douglas RG. The effect of chlorhexidine mouthwash on bacterial microcolonies in recurrent tonsillitis. Aust J Otolaryngol 2021;4:27.


