
Fungal otitis externa and tympanic membrane perforation: four-year experience at a Victorian hospital
Introduction
Fungal otitis externa (FOE), also known as otomycosis, is a superficial infection of the skin of the external auditory canal due to fungal pathogens. It is reported that 9% of all cases of otitis externa are due to fungal pathogens, most commonly Aspergillus or Candida species (1,2). Tympanic membrane perforation (TMP) due to FOE is a known but infrequently reported complication. The mechanism of perforation due to fungal infection has been attributed to mycotic thrombosis of vessels causing avascular necrosis of the tympanic membrane (3). A recent literature review by Koltsidopoulos et al. (2) found the rate of TMP secondary to FOE to be between 0–16.67% in eight studies. The reported rate of persistent TMP ranged from 5.5–27% in three studies. The management of FOE with TMP remains a challenge due to the ototoxic potential and middle ear irritation from topical antifungal agents or solvents used in their preparation (2). There are no human studies to confirm ototoxic potential of commonly used agents, however animal studies suggest clotrimazole, miconazole and tolnaftate are potentially safer choices (4). Hence, the aims of our study are to review the incidence of FOE with TMP and recurrence rates, as well as our management practices in a specialty ear, nose, and throat emergency department (ED).
The Royal Victorian Eye and Ear Hospital (RVEEH) is a dedicated hospital for both ophthalmology and otolaryngology patients with a 24-hour ED. Over a four-year period, there were over 10,000 presentations to the ED for otitis externa. The emergency department clinical practice guideline (CPG) for FOE was finalized in 2017 (5) and specific guidelines for when a TMP is present is seen in Table 1.

Full table
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/ajo-21-10).
Methods
A retrospective chart review was performed with a search using diagnostic codes “otitis externa” and “tympanic membrane perforation” for emergency presentations between June 2015 and October 2019. As there was no diagnostic code for FOE, diagnosis of FOE was accepted on the documentation of fungal spores on examination or positive culture on swab. Diagnosis of TMP was confirmed on documented examination finding.
Data collection included patient demographics, risk factors for otitis externa, perforation details (written or diagrammatic description), treatment and complications during follow-up (persistent TMP, recurrence of FOE with or without TMP after documentation of resolved infection). Resolution of TMP was accepted on clinical examination with or without tympanogram.
Statistical analysis
The statistical analysis was performed using JASP 0.11.1 (6). Continuous variables were summarised using mean ± standard deviation (SD) or median (interquartile range) depending on the underlying distribution of the data. Categorical data was reported in number and percentage. To compare acute TMP and chronic TMP groups, the Chi-square test was used to compare categorical data, and Mood’s Median test was used to compare medians of continuous data. P value of <0.05 was regarded as statistically significant.
Formal ethics approval was not required as the study was primarily a clinical audit with no identifiable patient data included. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
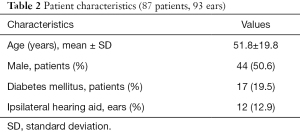
A total of 93 ears in 87 patients with FOE and TMP were identified between June 2015 and October 2019. Mean age was 51.8 years and 44% were male. Seventeen (19.5%) patients had diabetes mellitus and 12 (12.9%) had ipsilateral hearing aid use. Median follow up time was 92.1 days and five patients were lost to follow up. Patient characteristics are summarised in Table 2.
Full table
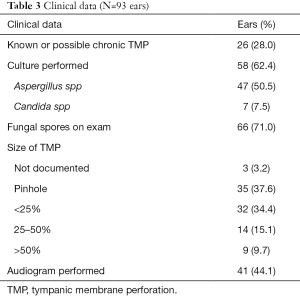
In 26 (28%) ears, there was a known or possible chronic TMP. In the remaining 67 ears, there was no history of previous TMP. Diagnosis was confirmed by culture in 58 (62.4%) ears and by documented visible fungal spores in 66 (71%) ears. Of the ears with documented fungal spores, there was a positive fungal culture in 33 ears. Size of TMP was predominantly pinhole (35 ears) or less than 25% (32 ears). Clinical data regarding diagnosis and characteristics of FOE and TMP are summarised in Table 3.
Full table
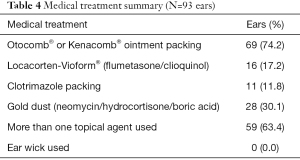
Medical management of the infection was most commonly Otocomb® or Kenacomb® (Triamcinolone acetonide/neomycin/gramicidin/nystatin) ointment packing (74.2% ears). “Gold dust” (neomycin/hydrocortisone/boric acid) was used in 30.1% of all ears. Locacorten-Vioform® (flumetasone/clioquinol) instillation was used less commonly and in 16 ears it was used despite ototoxic potential in the setting of a known TMP. More than one topical agent was used in 59 ears (63.4%). The medical management is summarised in Table 4.
Full table
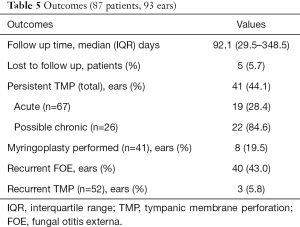
Median follow-up time was 92.1 days. Persistent TMP was noted in 41 ears (44.1%), of which, 19 were in ears with acute TMP and 22 were in ears with history of chronic TMP. A myringoplasty was performed in eight ears with persistent TMP (8/41, 19.5%), of which four were in ears with acute TMP and the remaining four in those with history of chronic TMP. In 40 ears (40/93, 43%), there was recurrence of fungal OE after documented resolution of infection. Out of 52 ears with documented spontaneous healing of TMP, there was a recurrence of FOE with TMP in three ears (3.2%). Outcomes are summarised in Table 5.
Full table
The acute and chronic TMP groups were compared, with results shown in Table 6. There were no significant differences in the rate of ipsilateral hearing aid use, median follow up time, recurrence of FOE, more than one topical agent used, or audiogram performed. The chronic TMP group had a significantly higher proportion of larger size TMP (P=0.005) and rate of persistent TMP (P<0.001).

Full table
Discussion
Our study represents, to date, the largest cohort of FOE with TMP and rates of persistent TMP. A total of 93 ears were included in the study, of which 67 ears were assumed to have an acute TMP secondary to FOE as the patient had no previous history of ear pathology, or a previously documented intact TM. Of these, the rate of persistent TMP was 28.4%, which is comparable to Song et al. (7) with a reported rate of 27%. However, their smaller cohort consisted of 11 patients, of which seven had no history of ear disease. The spontaneous closure rate of 71.6% in the acute TMP group is comparable to studies of traumatic TMP, with reported rates of spontaneous closure in 67.5–97% (8-10). The rate of persistent TMP in the chronic TMP group was significantly higher compared with the acute TMP group (P<0.001). This suggests that these groups represent different disease states. Those with chronic TMP or chronic suppurative otitis media may have impaired eustachian tube function, increased ear canal moisture or humidity or chronic use of antibacterial ear drops which predispose to fungal infection and persistent TMP.
The size of the TMP was predominantly small (pinhole or less than 25% surface area of the TM) which improves the chance of spontaneous closure (8,11,12). There was a significant difference between TMP size in the acute TMP group with a greater proportion of pinhole and <25% size compared to the chronic TMP group (P=0.005). Hurst (3) reported the size of perforations was less than one eighth (12%) of the total TM area in the majority of 22 patients. Song et al. (7) and Ho et al. (13) did not report on the size of the TMP.
Clinical Practice Guidelines (CPG) recommendation at the RVEEH when TMP is present in cases of otomycosis is seen in Table 1 (5). Concordant with the guidelines, our study found that Otocomb®/Kenacomb® ointment packing was the most common treatment used after thorough ear toilet. Locacorten-Vioform® was intentionally used in 16 ears with a perforation despite ototoxic potential. In these cases, the patient was generally non-responsive to usual treatment regimens with regular aural toilet and instillation of antifungal ointments or creams. Patients are counselled regarding ototoxic potential; however, an audiogram is not routinely performed before and after a course of Locacorten-Vioform®. In addition, an audiogram is not routinely used to confirm the presence or absence of a TMP in our institution and was performed in only 44.1% of all cases in our cohort. There was no significant difference in audiogram rate between acute and chronic TMP groups.
An ear swab for culture is not routinely used in the management of otitis externa. The RVEEH CPG recommends a swab only if otitis externa is not improving after the initial presentation (5). In this group, 64.2% of ears had the diagnosis confirmed on ear culture. As reported in most otomycosis studies, the most common pathogens isolated were Aspergillus, specifically Aspergillus niger, and Candida species.
In our institution, gold dust powder is generally used to prevent recurrence of infection. It is instilled into the ear using a speculum and pneumatic otoscope. In nearly two thirds of patients, more than one topical agent was used to treat FOE. In addition, there was a considerable rate of recurrent fungal infection (43%), which is consistent with the tenacious nature of this disease and difficulty with managing these patients.
It is widely accepted that difficulty with conservative management of FOE stems from the potential of middle ear irritation and ototoxicity of antifungal solutions (2). Indeed, the recommended RVEEH CPG treatment of Otocomb®/Kenacomb® still carries ototoxic potential due to possible migration of the ointment through the TMP and into the middle ear. Our CPG recommends painting, rather than filling, the canal with ointment in cases of large TMP to reduce the volume of ointment within the ear canal and therefore the chance of ototoxicity. It is not recommended that the ointment is placed directly in the middle ear through the perforation. Alternative therapies have been proposed to prevent this, which include placement of a gauze wick saturated with antifungals (3,14), patient self-administered Q-tip with clotrimazole (14) and sterile carbon paper patch closure of the TMP combined with application of Castellani’s solution to the ear canal (15). Ear wicks were not used in our institution over the study period. It is noted that standard expandable ear wicks have been found to have poor rates of absorption and expansion with oil-based ear drops (Canesten®, Locacorten-Vioform®, ichthammol glycerin) (16). Aqueous ear drops are preferred such as acetic acid (16) or a gauze wick as described in previously cited studies (3,14).
Due to the retrospective nature of our study, there were limitations regarding documentation and data collection. Identification of cases relied on the accuracy of coding during the ED presentation and therefore missed cases are inevitable. There are no separate codes to distinguish otitis externa due to a bacterial or fungal source. Therefore, the true rate of FOE and incidence of FOE with TMP cannot be established in this study. Case series have reported the rates of FOE with TMP as between 3–16.7% (2). The size of the TMP was reported in written notes or with a diagram, which is prone to inter-clinician and intra-clinician variability. Perforations involving the malleus or in the posterosuperior location have been reported as a risk factor for delayed healing (17). The location of the TMP was not examined in this study as it was not clearly documented. Duration of antimicrobial treatment was not reviewed in this study, however initial antimicrobial treatment duration in our institution is two weeks with further review, aural toilet, and reassessment for continuing treatment. Therefore, the impact of treatment time on the likelihood of persistent perforation and the rate of infection recurrence was not investigated.
Conclusions
TMP due to FOE is an infrequent but important complication for clinicians to be aware of as it may complicate management with usual antifungal agents. Recommendations for management of FOE with TMP is aural toilet with instillation of viscous antifungal ointment to prevent passage into the middle ear space. When TMP is due to FOE, it is likely to be of smaller size and is less likely to persist after infection has been successfully treated.
Acknowledgments
Health Information Services, The Royal Victorian Eye and Ear Hospital.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/ajo-21-10
Data Sharing Statement: Available at https://dx.doi.org/10.21037/ajo-21-10
Peer Review File: Available at https://dx.doi.org/10.21037/ajo-21-10
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/ajo-21-10). JWJL receives medical writing and Data collection support. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Formal ethics approval was not required as the study was primarily a clinical audit with no identifiable patient data included. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Viswanatha B, Sumatha D, Vijayashree MS. Otomycosis in immunocompetent and immunocompromised patients: comparative study and literature review. Ear Nose Throat J 2012;91:114-21. [Crossref] [PubMed]
- Koltsidopoulos P, Skoulakis C. Otomycosis With Tympanic Membrane Perforation: A Review of the Literature. Ear Nose Throat J 2020;99:518-21. [Crossref] [PubMed]
- Hurst WB. Outcome of 22 cases of perforated tympanic membrane caused by otomycosis. J Laryngol Otol 2001;115:879-80. [Crossref] [PubMed]
- Tom LW. Ototoxicity of common topical antimycotic preparations. Laryngoscope 2000;110:509-16. [Crossref] [PubMed]
- Vartanyan M, Orimoto K, Crock C, et al. Eyeandear.org.au. Available online: https://www.eyeandear.org.au/content/Document/CPG/Fungal%20Otitis%20Externa%20Clinical%20Practice%20Guideline.pdf
- JASP Team (2020). JASP (Version 0.14.1) [Computer software]. Available online: https://jasp-stats.org/
- Song JE, Haberkamp TJ, Patel R, et al. Fungal otitis externa as a cause of tympanic membrane perforation: a case series. Ear Nose Throat J 2014;93:332-6. [Crossref] [PubMed]
- Jellinge ME, Kristensen S, Larsen K. Spontaneous closure of traumatic tympanic membrane perforations: observational study. J Laryngol Otol 2015;129:950-4. [Crossref] [PubMed]
- Tachibana T, Kariya S, Orita Y, et al. Spontaneous closure of traumatic tympanic membrane perforation following long-term observation. Acta Otolaryngol 2019;139:487-91. [Crossref] [PubMed]
- Lou ZC, He JG. A randomised controlled trial comparing spontaneous healing, gelfoam patching and edge-approximation plus gelfoam patching in traumatic tympanic membrane perforation with inverted or everted edges. Clin Otolaryngol 2011;36:221-6. [Crossref] [PubMed]
- Lou ZC, Tang YM, Yang J. A prospective study evaluating spontaneous healing of aetiology, size and type-different groups of traumatic tympanic membrane perforation. Clin Otolaryngol 2011;36:450-60. [Crossref] [PubMed]
- Sogebi OA, Oyewole EA, Mabifah TO. Traumatic tympanic membrane perforations: characteristics and factors affecting outcome. Ghana Med J 2018;52:34-40. [Crossref] [PubMed]
- Ho T, Vrabec JT, Yoo D, et al. Otomycosis: clinical features and treatment implications. Otolaryngol Head Neck Surg 2006;135:787-91. [Crossref] [PubMed]
- Abou-Halawa AS, Khan MA, Alrobaee AA, et al. Otomycosis with Perforated Tympanic Membrane: Self medication with Topical Antifungal Solution versus Medicated Ear Wick. Int J Health Sci (Qassim) 2012;6:73-7. [Crossref] [PubMed]
- Görür K, İsmi O, Özcan C, et al. Treatment of Otomycosis in Ears with Tympanic Membrane Perforation is Easier with Paper Patch. Turk Arch Otorhinolaryngol 2019;57:182-6. [Crossref] [PubMed]
- Clamp PJ. Expansile properties of otowicks: an in vitro study. J Laryngol Otol 2008;122:687-90. [Crossref] [PubMed]
- Lou ZC, Lou ZH, Zhang QP. Traumatic tympanic membrane perforations: a study of etiology and factors affecting outcome. Am J Otolaryngol 2012;33:549-55. [Crossref] [PubMed]
Cite this article as: Tan H, Lim JWJ, Rose E. Fungal otitis externa and tympanic membrane perforation: four-year experience at a Victorian hospital. Aust J Otolaryngol 2021;4:28.


