Paediatric sinonasal surgery: a literature review
Introduction
Sinonasal pathology is common in children yet, has a unique pathology, symptomatology, management considerations and implications when compared to sinonasal disease in adults. Surgery and medical therapy in children with sinonasal pathology is controversial and is less standardised when compared to that in adults. A consensus on the indications for sinonasal surgery in children is also lacking. Ear, nose and throat surgeons are often reluctant to perform procedures such as septoplasty, turbinate or endoscopic sinus surgery in children (1). This reluctance stems from a concern that sinonasal surgery in children will negatively impact facial growth and also the difficulties of post-operative care in children. The following article is presented in accordance with the narrative review reporting checklist (available at https://dx.doi.org/10.21037/ajo-21-16).
Objectives
This paper aims to explore and present the developmental, anthropometric, anatomical and pathophysiological differences between adult and paediatric sinonasal anatomy, physiology and pathology. The role of effective and safe surgical treatment of these disorders in children will then be presented with key considerations and recommendations highlighted.
Methods
A structured literature search was performed using Medline, PubMed and Embase from inception to June 20, 2021. The search was restricted to English language articles only and search terms related to topics of paediatric sinonasal pathology, anatomy pathophysiology and surgery were identified and queried to identify relevant articles. The following terms were used ‘pediatrics’, ‘cephalometry’, ‘craniofacial morphology’, ‘craniofacial abnormalities’, ‘nasal obstruction’, ‘septoplasty’, ‘rhinosinusitis’, ‘inferior turbinate’ and ‘endoscopic sinus surgery’. Articles were reviewed for inclusion by the author and references of relevant articles were then hand-searched to identify additional manuscripts. Relevant websites were also included.
Results
A total of 1,701 papers were initially identified and 1,531 papers were excluded due to lack of relevance or duplication. Thirty-six other papers that were relevant to the topic were identified from a hand search of the references. Six other references were discovered from websites, one from a clinical trials registry and two from book chapters relevant to the topic. One reference found during the hand-search of the references was translated from French by the author.
The importance of nasal breathing on respiration
The nose contributes more than 50% of the total resistance of the upper airway and is the physiologic route of respiration during sleep as it is more efficient than mouth breathing (2). Nasal resistance is higher in children than in adults and demonstrates growth-dependent changes up to the age of 19 years (3). In fact, the nasal resistance in a 7-year-old child is double that of an adult (3).
Nasal airflow stimulates respiration and improves upper airway dilatory muscle tone via the nasal-ventilatory reflex (4,5). Evidence for this reflex is supported by studies that demonstrated an increase in contralateral thorax amplitudes with unilateral nasal obstruction and ipsilateral thorax expansion when air was blown into one nostril (6,7). Another prospective controlled study found that stimulation of the middle meatal mucosa in healthy volunteers results in a reduction in forced expiratory volume in the first second (FEV1) and an increase in total pulmonary resistance (8). This effect disappeared when the mucosa was anaesthetised (8). Further, nasal airflow also stimulates the trigemino-hypoglossal reflex. Changes in nasal intraluminal pressure and airflow will stimulate afferent signals from nasal mucosal trigeminal mechanoreceptors to the medulla and then to the genioglossus muscle via the hypoglossal motor neuron, resulting in tongue protrusion and improved airway patency (9-12). This reflex may explain the finding of increased upper airway collapsibility when the nasal mucosa is anaesthetised (13). Therefore, dynamic changes in the cross-sectional area of the nose will alter airflow velocity, resulting in the altered stimulation of airway mechanoreceptors and downstream modulation of the downstream collapsible pharyngeal segment of the upper airway (2). Further, a change from laminar flow to turbulent flow in the nose (for example with inferior turbinate hypertrophy or nasal septal deviation) will dehydrate nasal mechanoreceptors and negatively impact their function (2).
The impact of prolonged nasal obstruction in children
Experimental studies in young Rhesus monkeys have demonstrated significant craniofacial and neuromuscular changes secondary to artificially-induced nasal obstruction (14-16). Nasal airflow is a stimulus for lateral maxillary growth and lowering of the palatal vault (17). Many human observational studies have also noted the craniofacial impacts of prolonged mouth breathing/nasal obstruction in children including increased upper and lower anterior facial height, mandibular and maxillary retrognathia, more proclined maxillary incisors, increased palatal height, a narrow, V-shaped maxillary arch, increased soft palate length and thickness and more cross bites (17-19). In fact, in a study assessing the impact of adenoid hypertrophy on facial morphology, it was found that the more severe nasal obstruction resulted in the most divergent cephalometric measures (20).
These craniofacial changes may also predispose a child to sleep disordered breathing in adulthood (21). Nasal obstruction is also an independent contributor to sleep disorders such as obstructive sleep apnoea syndrome (22). Chronic mouth breathing in children causes a downward and posterior movement of the mandible and hyoid bone which results in an inferior and posterior location of the genioglossus muscle further narrowing of the upper airway (2,19,23). In children, the impact of sleep disordered breathing cannot be underestimated as “untreated chronic sleep disorders may lead to impaired brain development, neuronal damage and permanent loss of developmental potentials” (24). Sleep disordered breathing in children has also been associated with impaired psychomotor and cognitive performance, daytime somnolence, enuresis and hyperactivity (25,26). This may be due to the impact of sleep disordered breathing during periods of critical brain development where the sleep needs are greatest and the fact that paediatric neuronal circuits are primed to acquire and respond to information yet, they more susceptible to injury (27-29). However, unlike adults, the upper airway in children is more mechanically stable, particularly at the level of the tongue and velopharynx (23). This is due to the more superiorly located hyoid bone and improved mechanical efficiency of the genioglossus muscle’s protrusor activity (23). This explains the extended neck position adopted by many children with sleep disordered breathing in an attempt to elevate the hyoid bone and increase the retrolingual airway space (23). Other consequences of craniofacial immaturity favouring a more stable upper airway in children include reduced nasomaxillary height, a shorter vertical height of the pharynx, a more obliquely oriented pharynx, shorter tongue height and a reduced angle formed by the posterior border of the mandible, angle of the mandible (gonion) and the most inferior point of the chin (gnathion) (23). This upper airway mechanical stability explains why relief of nasal obstruction in children can actually cure their sleep-disordered breathing, in contrast to adults in whom relief of nasal obstruction rarely cures significant sleep-disordered breathing (23). Prolonged nasal obstruction in children has also been found to be associated with an increased incidence of respiratory tract infections including bronchitis and sinusitis (30).
Paediatric sinonasal anatomy
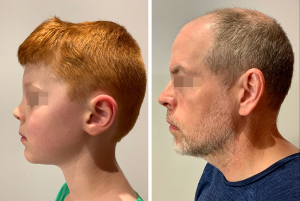
Children’s noses are less projected, shorter and more cartilaginous when compared to adults (31). Compared to adults, children have a larger nasolabial angle, shorter nasal dorsum, a flatter tip with a shorter columella (Figure 1) (32). Children also have wider and more rounded nares (3). Whilst the nose continues to grow into older age, nasal and midfacial maturation (defined as the steepest descent in growth velocity) is complete by the age of 14–20 years in females and 16–25 years in males (33,34). The maximum growth velocity of the Caucasian nose is between 8–13 years (35). Nasal and facial growth velocities in children also relate to body height velocity (36). An anthropometric study of 280 people noted the following anthropometric milestones:
- Nasal height and bridge length reaches maturity at the age of 15 years in males and 12 years in females (37).
- The nasal dorsum and anterior to posterior depth reaches maturity at 14 years in males and 12 years in females (37).
- Nasal tip protrusion reaches maturity at 15 years in males and 13 years in females (37).
Nasal septum
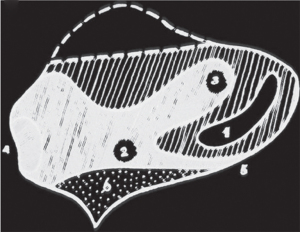
Between 37% and 57% of neonates are born with some form of septal deformity (38). The nasal septum is cartilaginous at birth, progressively ossifies over time and continues to grow until up to 36 years of age, particularly in the central section and the anterior free end (31,39,40). As the paediatric septum is predominantly cartilaginous, children are more prone to greenstick and avulsion fractures, and also septal haematomas, following mild trauma which can also more easily result in splaying and disarticulation of the nasal bones (31,41,42). The septum undergoes a rapid growth phase immediately after birth until 2 years of age where the cartilaginous septum reaches adult dimensions (39). Subsequent septal growth decelerates and is predominantly bony (39). Continued septal growth beyond the growth period of the surrounding facial skeleton may contribute the greater magnitudes of septal deviation in adults (43). The bony septum comprises the vomer and the perpendicular plate of the ethmoid bone. The perpendicular plate undergoes endochondral ossification and the vomer undergoes intramembranous ossification (39). Endochondral ossification is much slower than intramembranous ossification therefore, much of the bony growth of the septum into adulthood is due to growth of the perpendicular plate of the ethmoid bone (39,44). The junction of the perpendicular plate of the ethmoid bone and cartilaginous septum is a key growth zone for the length and height of the septum and nasal dorsum (32). However, the junction of the cartilaginous septum and vomer is not a key area for nasal growth (32). The nasal septum contains two thicker areas corresponding to growth zones: the sphenodorsal and sphenospinal zones (Figure 2) (32). The sphenodorsal zone controls development of the length and height of the nasal dorsum and the sphenospinal zone is responsible for the forward projection of the premaxilla and maxilla and sagittal growth of the septum (39). A saddle-nose deformity will result from injury to the sphenodorsal zone (Figure 3) and injury to the sphenospinal zone will result in impaired growth of the premaxilla and midface (45). Unlike in adults, septal cartilage regeneration after injury is possible in children, yet requires intact perichondrium (46).
The anterior septospinal ligament anchors the septum to the premaxilla in the midline and contributes to outgrowth of the nasal dorsum by placing tension on the premaxillary suture inducing an osteogenic response (32,47,48). This process is known as the nasal septal traction model (49). Early fusion of the premaxillary suture results in an increase in anterior nasal spine development and septal deviations possibly due to the loss of the impact of ligamentous tension on the premaxillary suture (49,50). A deviated septum has been found to be associated with an anterior displacement of the inferior aspect of the anterior sphenoid body (corresponding to the sphenoethmoidal synchondrosis, a key growth centre) and also with a dorsal rotation of the midline skull base (43). It is therefore, imperative to preserve the septospinal ligament when performing septal surgery in children.
Inferior turbinates
The inferior turbinates develop from the maxilla and lateral cartilaginous capsule on the lateral nasal wall, whereas the middle and superior turbinates develop from the ethmoid bone on the roof of the nasal cavity and septal wall, becoming more lateral during development (51). The inferior turbinate has its own ossification centre and ossifies during the fifth embryonic month (52,53). Therefore, surgery on the inferior turbinate does not impact the development of other facial bones.
Paranasal sinuses
The paranasal sinuses in children continue to develop until early adulthood and reach maturity at different rates (51). Pathologic processes that occur during these developmental stages can impact paranasal sinus development (54). Whilst paranasal sinus development begins in the second to third month of gestation, only the maxillary and ethmoid sinuses are present on imaging at birth (54-56). However, these sinuses may not be aerated at birth (57). The maxillary sinuses undergo a biphasic pneumatization process commencing with rapid pneumatization between the ages of 12 months and 4 years and the second phase coinciding with the descent of permanent dentition between the ages of 7–12 years (54,58,59). The floor of the maxillary sinus remains above the level of the nasal cavity floor until the age of 10–14 years (54,59). Thus, developing teeth can be injured during any maxillary sinus procedure performed prior to this age without appropriate caution.
The ethmoid sinuses develop from the cartilaginous olfactory capsule and are phylogenetically, anatomically, embryologically and functionally distinct from the other paranasal sinuses which develop beyond the confines of the olfactory capsule (59). Essentially, the size of the ethmoid sinus is constrained, whereas the remaining sinuses develop to a highly variable extent (59). Ultimately, the ethmoid sinuses comprise a system of between 6–16 air cells per side (59). The ethmoid sinuses are composed of distinctly thin-walled lamellae compared to the remaining sinuses that may contain more rigid and robust septations, not lamellae (59). This permits migration of ethmoid cells into surrounding bones or sinuses, resulting in a complex labyrinth-like structure occupying all available space (59). The ethmoid sinus cells can impact the ventilation and drainage of all the other sinuses.
The sphenoid sinuses commence pneumatisation between 1 and 3 years of age and are not really appreciable before the age of 3 years (56,58). Sphenoid sinus pneumatisation continues into the teenage years and may continue into adulthood (58). The sphenoid sinus does not become clinically relevant until the age of 10 years (58). As the posterior ethmoid sinus commenced pneumatisation earlier, it can grow above the sphenoid sinus, forming an Onodi cell (58).
The frontal sinuses only start to significantly pneumatise by the age of 4–8 years reaching adult size by 12 years or older (56,58,60). Frontal sinus pneumatisation results in a significant change in facial features during puberty. The frontal sinuses pneumatise into the frontal bone and therefore, increase the size of the skull base (51,59). This growth supports the palate which is growing to accommodate the increasing number of teeth as permanent dentition develops (51). Clinically, frontal sinusitis is unlikely prior to the age of 4 years (58). Further, frontal trephination should not be performed until the frontal sinus has pneumatised over the orbital plate or further to avoid intracranial penetration.
Adenoids
Adenoiditis is also a contributor to paediatric chronic rhinosinusitis (PCRS) (61,62). The adenoids grow until the age of 5–7 years and start to involute by the age of 12 years and therefore, are less likely to be a contributing feature in CRS in older children (63). The nasopharynx has a distinct microbiota and a greater bacterial diversity compared to the nasal cavity suggesting they are two distinct ecological sites despite being in anatomic continuity (64). Large adenoids contribute to sinus disease by serving as a bottleneck for the drainage of sinus secretions and also adenoids of any size may serve as a bacterial biofilm reservoir as the bacterial isolation rate from the adenoids increases in association with rhinosinusitis severity but not with adenoid size (65-68). Children with PCRS are more likely to have adenoid biofilms than those with obstructive sleep apnoea (68,69). Adenoid hypertrophy is also associated with divergent cephalometric measures and adenoidectomy prior to the age of 6 years has been recommended in children with adenoid hypertrophy to have the greatest impact on aberrant craniofacial growth (20,23).
Chronic rhinosinusitis in children
Rhinosinusitis is prevalent in children yet, is poorly understood. Of the 3–8 annual viral upper respiratory tract infections experienced by children, up to 10% of these will progress to acute bacterial rhinosinusitis (70-74). The overall prevalence of PCRS is up to 8% and this tends to reduce between the ages of 7–9 years (75,76). However, it is difficult to obtain accurate prevalence data on PCRS due to the difficulty in examining and obtaining accurate histories from children, physicians’ reluctance to perform imaging in children (due to concerns for radiation exposure and/or the need for sedation or general anaesthesia) and the frequent viral respiratory tract infections encountered by children that can mimic PCRS. Adenotonsillar hypertrophy may also cause symptoms similar to PCRS (77).
Paediatric rhinosinusitis is defined similarly to that in adults with the exchange of cough for olfactory dysfunction (see Symptoms below) (78). Paediatric CRS can significantly impact quality of life, concentration and academic performance (79).
Whilst PCRS with nasal polyposis is a distinct patient subgroup with a unique pathophysiology, the exact prevalence of PCRS with nasal polyposis is not known (77). Paediatric CRS with nasal polyps is less common than in adults yet, is present in up to 50% of children with PCRS undergoing sinus surgery (74,77,80). Paediatric CRS with polyps is uncommon in children under 10 years of age and is mostly found in children with allergic fungal sinusitis, cystic fibrosis or primary ciliary dyskinesia (77,81). Contributing factors in PCRS include familial risk, age, environmental exposures (cigarette smoking etc.), asthma and adenoiditis (61,62,82-85). The association of PCRS with atopy is controversial with many studies finding a prevalence similar to the general population and some reporting a high prevalence of atopy in PCRS (86-89). A retrospective review of 82 children with PCRS noted that central compartment atopic disease (CCAD), a variant of CRS strongly associated with atopy, was present in 38% (89-91). However, due to the heterogeneity in the definitions of PCRS and atopy, and varying geographic locations of the study populations, it cannot be determined whether atopy has any association with PCRS.
Chronic rhinosinusitis in general has a more complex pathophysiology compared to acute rhinosinusitis and PCRS has a different pathogenesis to adult CRS (77,92,93). The histopathology, immunology, microbiology, developmental anatomy, the incidence of gastroesophageal reflux disease and the presence of adenoids all contribute to this difference. The sinonasal mucosa in CRS differs between adults and children (92-94). The mucosa in children with PCRS demonstrates a different inflammatory response: the mucosa has less basement membrane thickening, less morphologic injury, less mucus gland hypertrophy and hyperplasia, is more lymphocytic and less eosinophilic than adults with CRS (92,94). A retrospective review of 82 children with PCRS noted that the serum eosinophil count was not elevated in any of the children with nasal polyposis (89). However, CCAD has been found to be slightly higher in older children (mean age 16.1 years) with CRS compared to adults with CRS in a retrospective cross-sectional study (89,95). The mucosa of children with PCRS also demonstrates different inflammatory and immune mediator profiles when compared to adults (96). In particular, cytokines such as IL6, IL8, IL12A and several TNF factors, noted to be elevated in adults with CRS, were found to be significantly decreased in children with PCRS in a prospective controlled study using gene microarray analysis in 21 children aged from 2.5–18 years (96,97). Further, IL5, IL6 and IL8, known to be elevated in certain adults with CRS, were not found to be elevated in children with PCRS (96,97). This study also found an upregulation of inflammatory genes that encode proteins involved in both the innate and the adaptive immune systems (96). A retrospective review of 86 children (aged 1–13 years) and 49 adults with recurrent sinus infections (>3 per year) noted that children were less likely to have humoral immunodeficiencies compared to adults (98). Older children with CRS tend to demonstrate what may represent a progression towards the adult histopathologic endotype and often demonstrate features common to both (94). This may be a reflection of a child’s developing immune system and sinuses and the involution of the adenoids among other factors. Another hypothesis is that the different inflammatory response in children with PCRS is due to a greater activation and/or dysregulation of the innate immune system and/or a shorter duration of inflammation (93). However, it must be considered that geographic variation may confound comparisons between adult and paediatric CRS (77).
There is a discordance between microbial abundance determined by culture and that detected by 16s ribosomal ribonucleic acid (rRNA) gene sequencing (64). This may explain the inconsistencies reported between studies. However, both culture and 16S rRNA studies have found that the microbiome in PCRS differs to that of adult CRS and that microbial diversity increases with age (64,99-101). This further supports the difference between PCRS and adult CRS. However, the flora of the middle meatus also differs between healthy children and adults and the microbiology in healthy children has been found to be similar to that of children with CRS (100,101). Certainly the microbiome in children differs between younger (4–12 years) and older (13–18 years) children (101). This may suggest that infection plays less of a role in PCRS.
The relationship between PCRS and gastroesophageal reflux disease (GORD) remains controversial, however, children do have a higher incidence of GORD than adults and up to 63% of children with CRS have been found to have GORD (102). Further, a prospective study reported an improvement in the symptoms of PCRS in children with GORD and PCRS who underwent treatment for GORD (102). Therefore, GORD should not be discounted in PCRS (64,103,104).
Overall, due to these differences, CRS in children under 12 years of age often differs to that in children over 12 years of age (55,74,101).
Symptoms
Unlike adults, cough is one of the most significant symptoms in PCRS and rhinorrhoea is one of the strongest predictors of PCRS (75,105). The combination of rhinorrhoea plus halitosis, cough, facial pain and nasal obstruction results in a near 100% probability of PCRS (75). Sleep disordered breathing or halitosis may be other more common chief complaints in children with PCRS (77).
Investigations
Children who do not respond to initial medical therapy or who present with complications of PCRS should undergo further investigations and examination. These investigations may include nasendoscopy (in children who will tolerate it), pathology, imaging, olfactory testing and measurements of nasal airflow and patency.
MRI scans may be indicated as an initial radiologic investigation (avoiding radiation) or in complicated sinus disease which has extended beyond the limits of the paranasal sinuses. However, MRI scans do not provide the detailed sinonasal bony anatomy and anatomic variants required prior to any sinus surgery. CT scans provide a high specificity and sensitivity for PCRS and are essential to assess sinonasal anatomy, extent of sinus development as well as the extent and severity of disease (74,106). Low radiation CT scans are available which expose children to doses as low as 0.1–0.2 mSv (107). Some in-office scanners provide CT scans with even lower doses (0.04–0.18 mSv) of radiation (108). These doses are comparatively low when compared to the annual radiation exposure due to background radiation of approximately 3.1 mSv (109). Whilst CT scans are a recommended aid to the diagnosis of PCRS, the radiation risks must be considered on an individual basis. It must also be noted that sinus disease has been found on CT scans in 18–45% of asymptomatic children undergoing scans for other indications (110-113). These findings must be interpreted with caution due to varying definitions of “abnormal” and many studies used non-validated scoring systems (110-112). The Lund-Mackay system was designed for scoring adult sinus disease on CT scans. This validated system scores the disease per sinus (the anterior and posterior ethmoid sinuses are scored separately) plus the osteomeatal complex from 0–2 with a total score from 0–24 being awarded (114). A score of ≥5 is considered indicative of CRS in adults (78). When this system was used and scaled using a correction factor for the number of sinuses present in an asymptomatic paediatric population, over 80% of children had sinus abnormalities on CT scan (115). However, the mean scaled score in these healthy patients was 2.81 suggesting a Lund-Mackay score of ≥4 is more likely to represent CRS in children (115). Another study found that a scaled Lund-Mackay score of ≥5 had a sensitivity of 86% and specificity of 85% for diagnosing PCRS (106). More recently, paediatric CT sinus staging system has been developed that considers both sinus opacification and the varying sinus developmental stages as a tool to predict which children may require surgery (116). However, this system did not demonstrate superiority to the Lund-Mackay system for distinguishing between disease severity (116). Finally, the maxillary and ethmoid sinuses are normally opacified at birth (57). These sinuses could be incorrectly diagnosed as abnormal if this fact is not considered.
Lateral airways x-rays to assess adenoid size are not recommended as adenoid size does not correlate with the adenoids functioning as a bacterial reservoir (65).
Pathology investigations may include a full blood count, vitamin D and IgE levels, biopsies, allergy testing and studies to exclude immunodeficiencies in certain children. Vitamin D3, a regulator of dendritic cell maturation and migration, has been found to be significantly lower in 90% of children with PCRS with polyps or allergic fungal sinusitis compared to healthy controls and children with PCRS without polyps (117). Vitamin D levels were also found to be lower in children with complications of acute rhinosinusitis (118). Vitamin D3 supplementation has been shown to reduce asthma-related hospitalisations and anti-inflammatory requirements in asthmatic children (119). Vitamin D3 levels have also been found to be inversely associated with the frequency of upper respiratory tract infections in people aged 12 years or older (120). However, the role for vitamin D supplementation in children with PCRS remains unclear.
Measures of nasal airflow and patency including peak nasal inspiratory flow, rhinomanometry and acoustic rhinometry are all possible in children with normative data available (3,121-125).
Patient reported outcome measures for children include the Sinus and Nasal Quality of Life Survey (SN-5 for children ≤5 years of age) (126). Or the Sinonasal Outcome Test (SNOT-22 for children >5 years of age).
Diseases such as atopy, cystic fibrosis, primary ciliary dyskinesia, allergic fungal sinusitis and immunodeficiencies (particularly IgA and IgG subclass deficiencies and an inadequate response to pneumococcal vaccination) should always be considered in children with PCRS, especially in those with nasal polyposis and/or a history of recurrent infections such as pneumonia (127,128). Allergic rhinitis is rare in children under 2–3 years of age (129-131). A nasal foreign body should be excluded in a child with unilateral symptoms. Asthma should also be investigated in children with PCRS as it is present in 18.1% of these children and 40.7% in children with PCRS and allergic rhinitis (132). It is also important to assess the ears in children with PCRS as there is a high concurrence of chronic otitis media with effusion and PCRS, particularly in children with PCRS under the age of 3 years (127,133-136).
Treatment
Medical therapy is the mainstay of treatment for PCRS with surgery reserved for those resistant to medical therapy or for complications such as periorbital abscesses. Yet, what constitutes optimal medical therapy for PCRS is yet to be determined and most recommendations are based on the efficacy of adult therapies. Allergen avoidance should be initiated in all children with allergic rhinitis. Immunotherapy should be considered in children with persistent allergic rhinitis that does not respond to avoidance and medical therapy. Saline irrigations or sprays are safe and effective in children as is the long-term use of second-generation intranasal corticosteroid sprays in children over the age of 3 years (137-139). Combination intranasal second-generation steroid and antihistamine sprays have demonstrated safety in children over 6 years of age (unpublished data) (140).
The bacteriology of PCRS is controversial, however, antibiotics may also be indicated for exacerbations of chronic rhinosinusitis or for complications of PCRS (74). Antibiotics alone are rarely beneficial in the treatment of PCRS and most recommendations are based on data from acute paediatric sinusitis studies (141-143). A recent prospective study using rRNA gene sequencing of nasopharyngeal and nasal cavity samples detected no significant difference in microbial diversity or composition between children with PCRS versus controls (64). Further, the risk of development of antimicrobial resistant bacteria increases in children who have had recent treatment with antibiotics and in those who attend daycare (144). However, when indicated, antimicrobial therapy should include antibiotics effective against anaerobic and beta-lactamase producing bacteria (141). Amoxycillin-clavulanate, a cephalosporin or clindamycin (for anaerobes) should be considered as first-line oral antibiotics when indicated in immunocompetent children (141,142,145). There is no consensus on the duration of antibiotic therapy, however, 20 days has been shown to be superior to 10 days. Parental therapy is reserved for children with significant complications or comorbidities. There is limited evidence or consensus supporting the routine use of antibiotic nasal irrigations in children and therefore, they are not recommended (74,141,142). Post-operative use of antibiotics irrigations may be of benefit in specific paediatric populations (e.g., those with cystic fibrosis), however, research is lacking in this area.
Systemic corticosteroids are reserved for severe disease or for complications of PCRS. The use of other therapies such as antihistamines, leukotriene receptor antagonists or imidazolines for PCRS is not routinely recommended unless warranted for concomitant disease such as allergic rhinitis or asthma (78). The use of imidazolines in children can cause toxicity and overdose in rare instances with doses of more than 2.5 mL or 0.4 mg/kg (146,147). Whilst children with gastroesophageal reflux disease do have an increased risk of sinusitis, empiric treatment of undiagnosed gastroesophageal reflux disease or extra-oesophageal reflux disease in PCRS is not recommended (74,148).
Surgical management
Surgery should be considered in children who fail medical management, for whom medical management is not an option or in those children with a significant complication of their disease.
Adenoidectomy
One meta-analysis of 9 moderate to good quality studies and a prospective interventional study of 60 children found that performing an adenoidectomy in children with PCRS improves quality of life and also improves the symptoms of PCRS (149,150). An adenoidectomy should be considered in any child with chronic rhinosinusitis to reduce the bacterial and biofilm load and has been recommended as a first line treatment for PCRS, particularly, in children under the age of 6 years (69,74,78). However, the success rates for adenoidectomy for PCRS vary from 50–92% and endoscopic sinus surgery has been found to result in greater symptom improvement versus adenoidectomy alone in these children (151,152).
Septoplasty
Septoplasty is commonly avoided in children due to concerns that septal surgery may negatively impact midfacial and nasal growth (153). This misconception stemmed from case reports and animal studies using aggressive septoplasty techniques (154,155). However, paediatric nasal septal surgery has been shown to significantly improve quality of life and to be effective and safe (31,156-161). In fact, adolescent growth can cause an exacerbation of deviated nasal structures if they are not addressed (162). If key areas or zones are respected, septal surgery does not impact facial growth (32,163). A recent systematic review included 8 papers and found no major disturbances in facial growth after septoplasty in children (164). They also concluded that both the external and endonasal approaches did not impair facial growth (164). However, they found the level of evidence for recommendations was level C due to the lack of a standardised method to measure facial growth, inadequate durations of follow-up, high losses to follow-up, a failure to report details of surgical techniques and a failure to evaluate growth of the whole face rather than that of the nose alone (164).
However, there is certainly enough evidence indicating the negative impact of nasal obstruction and nasal septal deviation on the nasal and midface growth and quality of life in children (18,158,165). Therefore, septoplasty should be considered when a septal deviation is contributing to nasal obstruction and when the benefits are determined to outweigh the risks.
Timing of septal surgery in children
Traditionally, it has been recommended to wait until a child is 5 or 6 years old or older before performing a septoplasty (157,166-168). However, a limited septoplasty using a unilateral mucoperichondrial flap in a 14-day-old child has been reported (169). The child, who had a severe anterior septal deviation, both obstructive sleep and awake apnoeas and cyanosis, demonstrated immediate resolution of the apnoeas post-operatively (169). Unfortunately, no long-term outcomes were presented (169). However, no facial disproportions were noted in two long-term follow-up studies after closed reduction of septal deviations performed in over 65 children at birth (170,171). Unfortunately, these studies did suffer from significant drop-out rates (170). Interestingly, a controlled study with long-term follow-up comparing neonates with septal deviations who did and did not undergo correction of their deviation within 1 week of birth (172). They noted that the subluxed caudal septum tended to be straight in both groups at 8-year follow-up, yet children with a subluxed body of the septal cartilage rarely straightened over time (172). However, persistent deviations were noted at 3 years of age in another similar long-term follow-up study of neonates with septal deviations who did not undergo treatment (170). No comment was made as to the location of the deviations. Successful septal surgery has also been reported in children as young as 4.5 years of age and this author has performed septoplasty in a 3-year-old child with cystic fibrosis and severe obstructive sleep apnoea with no cosmetic impairment at 3-year follow-up and complete resolution of the sleep apnoea (158). Therefore, if a child has a significant septal deviation causing severe airway obstruction, a septoplasty or closed reduction should be considered at any age. However, based on the limited evidence available, if the airway obstruction is not severe, waiting until the child is 4–5 years of age would be recommended.
Key points in paediatric septal surgery:
- Uni- or bilateral elevation of the mucoperichondrium does not impact facial growth, however, the mucosa of the nasal floor should not be elevated to prevent injury to the incisive nerves (32,173).
- Avoid incisions through the sphenopinal and sphenodorsal zones of the septum (32).
- Avoid separating the cartilaginous septum from the perpendicular plate of the ethmoid bone (32).
- There is a highly variable ossification of the skull base and septum in children and caution must be exercised when instrumenting the superior septum (32).
- The anterior edge of the perpendicular plate of the ethmoid bone is located intracranially in young children and becomes extracranial as they grow (32).
- Avoid resection of the anteroinferior portion of the septum to avoid injury to the septospinal ligament (173).
- Avoid perforation by re-inserting autologous cartilage (173).
- Resecting the junction of the vomer and septal cartilage does not interfere with nasal or midface growth (32).
Inferior turbinate reduction
A randomised, double-blinded study found that inferior turbinate reduction using the medial flap technique provides the best long-term results when compared to tissue ablative techniques (174). Two retrospective cohort studies of 107 children (1.2–17.9 years of age) and 227 children under 10 years of age concluded that inferior turbinate surgery is safe and effective in children and does not impair facial growth with up to 7 years follow-up (175,176). Any child with inferior turbinate hypertrophy should also be investigated for allergic rhinitis (177).
Balloon sinus catheterisation
Multiple prospective cohort studies have reported successful outcomes using balloon sinuplasty/catheterisation (BCS) in children with PCRS, often in combination with other procedures such as adenoidectomy (178-180). A systematic review also found BCS was effective and safe (181). However, the review was limited by the limited number of included studies and also as most of the studies were observational (181). A retrospective cohort blinded chart review and a blinded randomised controlled trial have not noted a difference between balloon sinuplasty and ESS or between balloon sinuplasty and adenoidectomy with maxillary sinus irrigation (182,183). Balloon dilatation is not without risks in the paediatric population due to the limited size of the nasal cavity and sinus ostia, the size of the balloons, the unique paediatric sinonasal anatomy and the radiation involved to confirm balloon placement. Due to these considerations, the conflicting results, the findings of a blinded randomised controlled trial, a lack of long-term outcomes and a lack of comparison data between balloon sinuplasty and ESS, balloon catheterisation in children with PCRS is not currently recommended.
Endoscopic sinus surgery
Further research into the indications and outcomes of endoscopic sinus surgery in children is required as many studies are retrospective, include small numbers, present poor quality data and rely on parental subjective reported outcomes. One retrospective chart review of 500 children aged 14 months to 16 years undergoing ESS describes exploring the frontal recess in all children with chronic or recurrent disease (184). The frontal sinuses do not commence pneumatisation before the age of 4–8 years and frontal sinus disease is rare in children under 4 years of age (56,58,60). Whilst detailed data regarding the age and number of the children specifically involved is not provided, the results of this study must be interpreted with caution. It is unfortunate that meta-analyses have included data from this study which may have caused significant bias due to the large number of patients involved (185,186). However, a systematic review that excluded the data from this study concluded that paediatric endoscopic sinus surgery was safe (with a complication rate of 1.4%), effective and resulted in long-term improvements in quality of life (187). A non-randomised prospective study of 22 children with sinonasal disease undergoing either ESS or adenoidectomy found that ESS resulted in a significantly greater improvement in quality of life (188). Another non-randomised controlled prospective study noted that SNOT-22, Lund-Mackay and Lund-Kennedy scores improved after ESS in 132 children with PCRS compared to 15 healthy controls (189). This study also found that the number of ciliated cells, ciliary beat frequency, cell viability and ciliary length were significantly higher 9 months after surgery than pre-operatively (189). Further, epithelial dystrophy and neutrophil infiltration were significantly reduced 12 months post-operatively (189). Interestingly, cilial function was noted to be severely impaired 0-3 months post-operatively (189). A prospective observational study of 39 children with PCRS with polyps (mean age 10.9 years) undergoing ESS demonstrated an improvement in quality of life after surgery (190). Similarly, a retrospective questionnaire-based review of 115 children undergoing ESS for PCRS noted sustained improvements in symptoms and quality of life with a mean follow-up of 5.4 years (191).
Regarding the impact of ESS on facial growth, one retrospective age-matched cohort study of 67 children and one longitudinal review of 8 children found that ESS did not impair facial growth (192,193). A retrospective review of 11 children undergoing endoscopic skull base surgery before the age of 7 and 33 patients who underwent surgery after this age also found no impact on midfacial growth (194).
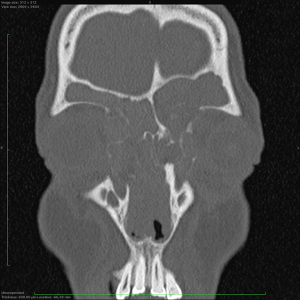
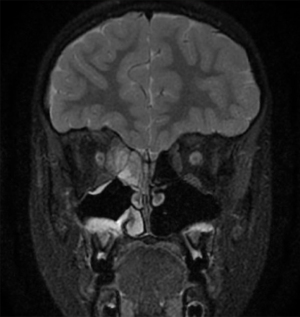
Antral irrigation combined with adenoidectomy is a simple procedure that may be considered in children with minimal sinus disease (78). More extensive sinus surgery should be considered in children who fail adenoidectomy and antral washout, in children with more severe disease such as allergic fungal sinusitis (Figure 4), primary ciliary dyskinesia, cystic fibrosis (Figure 5) or immunodeficiencies or in children with complications of CRS such as large subperiosteal abscesses extending into the orbit (Figure 6).
Patients with nasal allergy and higher Lund-Mackay scores have been shown to be at risk for worse outcomes after ESS, whilst older age is a positive prognostic factor (195). However, pre-operative management of atopy in children with PCRS improves ESS outcomes (196). Certainly, children with asthma and PCRS are more likely to benefit from ESS (152). A prospective observational study of 14 children (3.5–13 years of age) reported that endoscopic sinus surgery reduced hospitalisations, corticosteroid requirements and absenteeism and improved symptom scores in children with asthma (197). No improvement in lung function was noted in this study, however, lung function in children with cystic fibrosis does improve after ESS (198). Complications and readmissions after ESS are more common in children undergoing ESS as an emergent procedure, those with a history of coagulation disorders, asthma or cystic fibrosis and those aged under 3 years (199,200).
The use of any packing material, let alone non-absorbable dressings, is generally not indicated in ESS, especially when mucosal preservation techniques are used (201-204). Further, frequent debridements have not been shown to provide any additional benefit to outcomes post-operatively, except to delay pulmonary exacerbations in patients with cystic fibrosis (205-209). It is the author’s experience that children as young as 4 are able to tolerate, and even self-administer, saline irrigations. Further, the use of videos of other children irrigating and allowing the child a period of acclimatization to the irrigation bottle prior to the surgery can improve compliance. This reduces the need for post-operative removal of dressings and the frequency of debridements in children which often requires sedation or a general anaesthetic. When required, a debridement under general anaesthetic is safe although, it is ideal to minimise the frequency. The impact of general anaesthesia in children has been shown to negatively impact academic performance in children exposed at the age of 37–48 months (210). This impact was less than that associated with gender, maternal education level and the child’s month of birth (210). Paediatric general anaesthesia has not been shown to negatively impact global development but does have a slight negative impact on manual dexterity and social communication skills (211).
Conclusion
Paediatric sinonasal surgery requires a detailed knowledge of nasal and facial growth patterns and of the unique age-related paediatric sinonasal anatomy. The pathophysiology and consequences of nasal obstruction and sinonasal disease in children differs to that of adults and even differs throughout the stages of childhood. The quality of the evidence for paediatric sinonasal surgery is predominantly level IV at best and is further limited due to inadequate follow-up periods which do not incorporate the second paediatric facial growth phase. However, when indicated, paediatric sinonasal surgery is safe, improves quality of life and should include adenoidectomy in children with PCRS, particularly those aged under 6 years. Finally, chronic nasal obstruction in children does result in craniofacial changes however, sinonasal surgery does not appear to impair facial growth, particularly, if certain key considerations regarding the septum and age-related sinus anatomy are respected. Future research is needed that includes randomised controlled trials, controlled trials and trials with extended follow-up periods that extend beyond the second facial growth phase.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/ajo-21-16
Peer Review File: Available at https://dx.doi.org/10.21037/ajo-21-16
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/ajo-21-16). The author serves as an unpaid editorial board member of the Australian Journal of Otolaryngology from Jan 2019 to Dec 2022. The author reports honoraria from Seqirus, Novartis and Medtronic. The author reports participation on Seqirus advisory board. The author is a general council member of Women in Rhinology Section of the American Rhinologic Society, an international Committee member of the North American Skull Base Society, a coordinator of MQ Health Skull Base Surgery Multidisciplinary Team and an education coordinator of Australia and New Zealand Skull Base Society. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All images of patients have been published with their consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cazzavillan A, Gaini RM, Pignataro L, et al. Treatment of rhinosinusitis: the role of surgery. Int J Immunopathol Pharmacol 2010;23:74-7. [PubMed]
- Shintaro C, Park CS. Establishing a Patent Nasal Passage in Obstructive Sleep Apnea. Sleep Med Clin 2019;14:41-50. [Crossref] [PubMed]
- Peksis K, Unger J, Paulauska S, et al. Relationships among nasal resistance, age and anthropometric parameters of the nose during growth. Rhinology Online 2018;1:112-21. [Crossref]
- Baraniuk JN, Merck SJ. Nasal reflexes: implications for exercise, breathing, and sex. Curr Allergy Asthma Rep 2008;8:147-53. [Crossref] [PubMed]
- Basner RC, Simon PM, Schwartzstein RM, et al. Breathing route influences upper airway muscle activity in awake normal adults. J Appl Physiol (1985) 1989;66:1766-71. [PubMed]
- Samzelius-Lejdstrom I. Researches with the bilateral troncopneumograph on the movements of the respiratory mechanism during breathing. Acta Otolaryngol (Stockholm) 1939;35:1-100.
- Sercer A. Investigations sur l’influence reflectoire de la cavite nasal sur le poumon du meme cote. Acta Otolaryngol 1930;14:82-90. [Crossref]
- Milicić D, Mladina R, Djanić D, et al. Influence of nasal fontanel receptors on the regulation of tracheobronchal vagal tone. Croat Med J 1998;39:426-9. [PubMed]
- Tankéré F, Maisonobe T, Naccache L, et al. Further evidence for a central reorganisation of synaptic connectivity in patients with hypoglossal-facial anastomosis in man. Brain Res 2000;864:87-94. [Crossref] [PubMed]
- Horner RL, Innes JA, Murphy K, et al. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol 1991;436:15-29. [Crossref] [PubMed]
- White DP, Edwards JK, Shea SA. Local reflex mechanisms: influence on basal genioglossal muscle activation in normal subjects. Sleep 1998;21:719-28. [Crossref] [PubMed]
- Lo YL, Jordan AS, Malhotra A, et al. Influence of wakefulness on pharyngeal airway muscle activity. Thorax 2007;62:799-805. [Crossref] [PubMed]
- Carberry JC, Hensen H, Fisher LP, et al. Mechanisms contributing to the response of upper-airway muscles to changes in airway pressure. J Appl Physiol (1985) 2015;118:1221-8. [PubMed]
- Miller AJ, Vargervik K, Chierici G. Experimentally induced neuromuscular changes during and after nasal airway obstruction. Am J Orthod 1984;85:385-92. [Crossref] [PubMed]
- Harvold EP, Tomer BS, Vargervik K, et al. Primate experiments on oral respiration. Am J Orthod 1981;79:359-72. [Crossref] [PubMed]
- Vargervik K, Miller AJ, Chierici G, et al. Morphologic response to changes in neuromuscular patterns experimentally induced by altered modes of respiration. Am J Orthod 1984;85:115-24. [Crossref] [PubMed]
- Gungor AY, Turkkahraman H. Effects of airway problems on maxillary growth: a review. Eur J Dent 2009;3:250-4. [Crossref] [PubMed]
- D'Ascanio L, Lancione C, Pompa G, et al. Craniofacial growth in children with nasal septum deviation: a cephalometric comparative study. Int J Pediatr Otorhinolaryngol 2010;74:1180-3. [Crossref] [PubMed]
- Harari D, Redlich M, Miri S, et al. The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients. Laryngoscope 2010;120:2089-93. [Crossref] [PubMed]
- Macari AT, Bitar MA, Ghafari JG. New insights on age-related association between nasopharyngeal airway clearance and facial morphology. Orthod Craniofac Res 2012;15:188-97. [Crossref] [PubMed]
- Guilleminault C, Stoohs R, Kim YD, et al. Upper airway sleep-disordered breathing in women. Ann Intern Med 1995;122:493-501. [Crossref] [PubMed]
- Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J Allergy Clin Immunol 1997;99:S757-62. [Crossref] [PubMed]
- Finkelstein Y, Wexler D, Berger G, et al. Anatomical basis of sleep-related breathing abnormalities in children with nasal obstruction. Arch Otolaryngol Head Neck Surg 2000;126:593-600. [Crossref] [PubMed]
- Jan JE, Reiter RJ, Bax MC, et al. Long-term sleep disturbances in children: a cause of neuronal loss. Eur J Paediatr Neurol 2010;14:380-90. [Crossref] [PubMed]
- Ali NJ, Pitson D, Stradling JR. Natural history of snoring and related behaviour problems between the ages of 4 and 7 years. Arch Dis Child 1994;71:74-6. [Crossref] [PubMed]
- Isaiah A, Ernst T, Cloak CC, et al. Association Between Habitual Snoring and Cognitive Performance Among a Large Sample of Preadolescent Children. JAMA Otolaryngol Head Neck Surg 2021;147:426-33. [Crossref] [PubMed]
- Hensch TK. Critical period regulation. Annu Rev Neurosci 2004;27:549-79. [Crossref] [PubMed]
- Miyamoto H, Hensch TK. Reciprocal interaction of sleep and synaptic plasticity. Mol Interv 2003;3:404-17. [Crossref] [PubMed]
- Galland BC, Taylor BJ, Elder DE, et al. Normal sleep patterns in infants and children: a systematic review of observational studies. Sleep Med Rev 2012;16:213-22. [Crossref] [PubMed]
- Bláhová O. Late results of nasal septum injury in children. Int J Pediatr Otorhinolaryngol 1985;10:137-41. [Crossref] [PubMed]
- Saniasiaya J, Abdullah B. Quality of life in children following nasal septal surgery: A review of its outcome. Pediatr Investig 2019;3:180-4. [Crossref] [PubMed]
- Verwoerd CD, Verwoerd-Verhoef HL. Rhinosurgery in children: developmental and surgical aspects of the growing nose. GMS Curr Top Otorhinolaryngol Head Neck Surg 2010;9:Doc05. [PubMed]
- Meng HP, Goorhuis J, Kapila S, et al. Growth changes in the nasal profile from 7 to 18 years of age. Am J Orthod Dentofacial Orthop 1988;94:317-26. [Crossref] [PubMed]
- Zankl A, Eberle L, Molinari L, et al. Growth charts for nose length, nasal protrusion, and philtrum length from birth to 97 years. Am J Med Genet 2002;111:388-91. [Crossref] [PubMed]
- van der Heijden P, Korsten-Meijer AG, van der Laan BF, et al. Nasal growth and maturation age in adolescents: a systematic review. Arch Otolaryngol Head Neck Surg 2008;134:1288-93. [Crossref] [PubMed]
- Flores-Mir C, Nebbe B, Major PW. Use of skeletal maturation based on hand-wrist radiographic analysis as a predictor of facial growth: a systematic review. Angle Orthod 2004;74:118-24. [PubMed]
- Akgüner M, Barutçu A, Karaca C. Adolescent growth patterns of the bony and cartilaginous framework of the nose: a cephalometric study. Ann Plast Surg 1998;41:66-9. [Crossref] [PubMed]
- Gray LP. Deviated nasal septum. Incidence and etiology. Ann Otol Rhinol Laryngol Suppl 1978;87:3-20. [Crossref] [PubMed]
- Van Loosen J, Van Zanten GA, Howard CV, et al. Growth characteristics of the human nasal septum. Rhinology 1996;34:78-82. [PubMed]
- Vetter U, Pirsig W, Helbing G, et al. Patterns of growth in human septal cartilage: a review of new approaches. Int J Pediatr Otorhinolaryngol 1984;7:63-74. [Crossref] [PubMed]
- Johnson MD. Management of Pediatric Nasal Surgery (Rhinoplasty). Facial Plast Surg Clin North Am 2017;25:211-21. [Crossref] [PubMed]
- Canty PA, Berkowitz RG. Hematoma and abscess of the nasal septum in children. Arch Otolaryngol Head Neck Surg 1996;122:1373-6. [Crossref] [PubMed]
- Goergen MJ, Holton NE, Grünheid T. Morphological interaction between the nasal septum and nasofacial skeleton during human ontogeny. J Anat 2017;230:689-700. [Crossref] [PubMed]
- Biga LM, S D, Harwell A, et al. Bone Formation and Development. Anatomy & Physiology: OpenStax and Oregon State University; 2020.
- Zimmermann CE, Troulis MJ, Kaban LB. Pediatric facial fractures: recent advances in prevention, diagnosis and management. Int J Oral Maxillofac Surg 2006;35:2-13. [Crossref] [PubMed]
- Pirsig W, Lehmann I. The influence of trauma on the growing septal cartilage. Rhinology 1975;13:39-46. [PubMed]
- Elayadath RR, Palakunnu BP. Septopremaxillary ligament traction system: a review. Int J Adv Med 2019;6:1363-71. [Crossref]
- Siegel MI, Mooney MP, Eichberg JW, et al. Septopremaxillary ligament resection and midfacial growth in a chimpanzee animal model. J Craniofac Surg 1990;1:182-6. [Crossref] [PubMed]
- Holton NE, Yokley TR, Figueroa A. Nasal septal and craniofacial form in European- and African-derived populations. J Anat 2012;221:263-74. [Crossref] [PubMed]
- Rönning O, Kantomaa T. Experimental nasal septum deviation in the rat. Eur J Orthod 1985;7:248-54. [Crossref] [PubMed]
- Keith A. The nasal cavities and olfactory structures. 4th ed. New York: Longmans, Green and Co; 1921.
- Georgakopoulos B, Le PH. Anatomy, Head and Neck, Nasal Concha. Florida: StatPearls Publishing; 2020.
- Neskey D, Eloy JA, Casiano RR. Nasal, septal, and turbinate anatomy and embryology. Otolaryngol Clin North Am 2009;42:193-205. vii. [Crossref] [PubMed]
- Lawson W, Patel ZM, Lin FY. The development and pathologic processes that influence maxillary sinus pneumatization. Anat Rec (Hoboken) 2008;291:1554-63. [Crossref] [PubMed]
- Badr DT, Gaffin JM, Phipatanakul W. Pediatric Rhinosinusitis. Curr Treat Options Allergy 2016;3:268-81. [Crossref] [PubMed]
- Pohunek P. Development, structure and function of the upper airways. Paediatr Respir Rev 2004;5:2-8. [Crossref] [PubMed]
- Kubal WS. Sinonasal anatomy. Neuroimaging Clin N Am 1998;8:143-56. [PubMed]
- Vaid S, Vaid N. Normal Anatomy and Anatomic Variants of the Paranasal Sinuses on Computed Tomography. Neuroimaging Clin N Am 2015;25:527-48. [Crossref] [PubMed]
- Márquez S, Tessema B, Clement PA, et al. Development of the ethmoid sinus and extramural migration: the anatomical basis of this paranasal sinus. Anat Rec (Hoboken) 2008;291:1535-53. [Crossref] [PubMed]
- Cohen O, Adi M, Shapira-Galitz Y, et al. Anatomic variations of the paranasal sinuses in the general pediatric population. Rhinology 2019;57:206-12. [Crossref] [PubMed]
- Neff L, Adil EA. What is the role of the adenoid in pediatric chronic rhinosinusitis? Laryngoscope 2015;125:1282-3. [Crossref] [PubMed]
- Belcher R, Virgin F. The Role of the Adenoids in Pediatric Chronic Rhinosinusitis. Med Sci (Basel) 2019;7:35. [Crossref] [PubMed]
- Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol 1979;133:401-4. [Crossref] [PubMed]
- Stapleton AL, Shaffer AD, Morris A, et al. The microbiome of pediatric patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2021;11:31-9. [Crossref] [PubMed]
- Shin KS, Cho SH, Kim KR, et al. The role of adenoids in pediatric rhinosinusitis. Int J Pediatr Otorhinolaryngol 2008;72:1643-50. [Crossref] [PubMed]
- Davcheva-Chakar M, Kaftandzhieva A, Zafirovska B. Adenoid Vegetations - Reservoir of Bacteria for Chronic Otitis Media with Effusion and Chronic Rhinosinusitis. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2015;36:71-6. [Crossref] [PubMed]
- Elwany S, El-Dine AN, El-Medany A, et al. Relationship between bacteriology of the adenoid core and middle meatus in children with sinusitis. J Laryngol Otol 2011;125:279-81. [Crossref] [PubMed]
- Zuliani G, Carron M, Gurrola J, et al. Identification of adenoid biofilms in chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol 2006;70:1613-7. [Crossref] [PubMed]
- Coticchia J, Zuliani G, Coleman C, et al. Biofilm surface area in the pediatric nasopharynx: Chronic rhinosinusitis vs obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 2007;133:110-4. [Crossref] [PubMed]
- Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics 2013;132:e262-80. [Crossref] [PubMed]
- Hamilos DL. Pediatric chronic rhinosinusitis. Am J Rhinol Allergy 2015;29:414-20. [Crossref] [PubMed]
- Lieser JD, Derkay CS. Pediatric sinusitis: when do we operate? Curr Opin Otolaryngol Head Neck Surg 2005;13:60-6. [Crossref] [PubMed]
- Aitken M, Taylor JA. Prevalence of clinical sinusitis in young children followed up by primary care pediatricians. Arch Pediatr Adolesc Med 1998;152:244-8. [Crossref] [PubMed]
- Brietzke SE, Shin JJ, Choi S, et al. Clinical consensus statement: pediatric chronic rhinosinusitis. Otolaryngol Head Neck Surg 2014;151:542-53. [Crossref] [PubMed]
- Leo G, Incorvaia C. The clinical spectrum of rhinosinusitis in children. Int J Immunopathol Pharmacol 2010;23:24-8. [PubMed]
- Sidell D, Shapiro NL, Bhattacharyya N. Obesity and the risk of chronic rhinosinusitis, allergic rhinitis, and acute otitis media in school-age children. Laryngoscope 2013;123:2360-3. [Crossref] [PubMed]
- Mahdavinia M, Grammer LC 3rd. Chronic rhinosinusitis and age: is the pathogenesis different? Expert Rev Anti Infect Ther 2013;11:1029-40. [Crossref] [PubMed]
- Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020;58:1-464. [Crossref] [PubMed]
- Cunningham MJ, Chiu EJ, Landgraf JM, et al. The health impact of chronic recurrent rhinosinusitis in children. Arch Otolaryngol Head Neck Surg 2000;126:1363-8. [Crossref] [PubMed]
- Hu KH, Lee FP, Cheng YJ, et al. Vascular endothelial growth factor and children featuring nasal polyps. Int J Pediatr Otorhinolaryngol 2007;71:23-8. [Crossref] [PubMed]
- Triglia JM, Nicollas R. Nasal and sinus polyposis in children. Laryngoscope 1997;107:963-6. [Crossref] [PubMed]
- Orb Q, Curtin K, Oakley GM, et al. Familial risk of pediatric chronic rhinosinusitis. Laryngoscope 2016;126:739-45. [Crossref] [PubMed]
- Anfuso A, Ramadan H, Terrell A, et al. Sinus and adenoid inflammation in children with chronic rhinosinusitis and asthma. Ann Allergy Asthma Immunol 2015;114:103-10. [Crossref] [PubMed]
- Reh DD, Higgins TS, Smith TL. Impact of tobacco smoke on chronic rhinosinusitis: a review of the literature. Int Forum Allergy Rhinol 2012;2:362-9. [Crossref] [PubMed]
- Sedaghat AR, Phipatanakul W, Cunningham MJ. Atopy and the Development of Chronic Rhinosinusitis in Children with Allergic Rhinitis. J Allergy Clin Immunol Pract 2013;6:689-691.e2. [Crossref] [PubMed]
- Leo G, Piacentini E, Incorvaia C, et al. Chronic rhinosinusitis and allergy. Pediatr Allergy Immunol 2007;18:19-21. [Crossref] [PubMed]
- Nguyen KL, Corbett ML, Garcia DP, et al. Chronic sinusitis among pediatric patients with chronic respiratory complaints. J Allergy Clin Immunol 1993;92:824-30. [Crossref] [PubMed]
- Tantimongkolsuk C, Pornrattanarungsee S, Chiewvit P, et al. Pediatric sinusitis:symptom profiles with associated atopic conditions. J Med Assoc Thai 2005;88:S149-55. [PubMed]
- Lee K, Kim TH, Lee SH, et al. Predictive Value of Radiologic Central Compartment Atopic Disease for Identifying Allergy and Asthma in Pediatric Patients. Ear Nose Throat J 2021; Epub ahead of print. [Crossref] [PubMed]
- White LJ, Rotella MR, DelGaudio JM. Polypoid changes of the middle turbinate as an indicator of atopic disease. Int Forum Allergy Rhinol 2014;4:376-80. [Crossref] [PubMed]
- DelGaudio JM, Loftus PA, Hamizan AW, et al. Central compartment atopic disease. Am J Rhinol Allergy 2017;31:228-34. [Crossref] [PubMed]
- Chan KH, Abzug MJ, Coffinet L, et al. Chronic rhinosinusitis in young children differs from adults: a histopathology study. J Pediatr 2004;144:206-12. [Crossref] [PubMed]
- Coffinet L, Chan KH, Abzug MJ, et al. Immunopathology of chronic rhinosinusitis in young children. J Pediatr 2009;154:754-8. [Crossref] [PubMed]
- Berger G, Kogan T, Paker M, et al. Pediatric chronic rhinosinusitis histopathology: differences and similarities with the adult form. Otolaryngol Head Neck Surg 2011;144:85-90. [Crossref] [PubMed]
- Hamizan AW, Loftus PA, Alvarado R, et al. Allergic phenotype of chronic rhinosinusitis based on radiologic pattern of disease. Laryngoscope 2018;128:2015-21. [Crossref] [PubMed]
- Wu X, Ghimbovschi S, Aujla PK, et al. Expression profiling of inflammatory mediators in pediatric sinus mucosa. Arch Otolaryngol Head Neck Surg 2009;135:65-72. [Crossref] [PubMed]
- Lennard CM, Mann EA, Sun LL, et al. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: response to systemic corticosteroids. Am J Rhinol 2000;14:367-73. [Crossref] [PubMed]
- Makary CA, Purnell PR, O'Brien D, et al. Antibody deficiencies are more common in adult versus pediatric recurrent acute rhinosinusitis. Am J Otolaryngol 2021;42:103004 [Crossref] [PubMed]
- Wagner Mackenzie B, Waite DW, Hoggard M, et al. Bacterial community collapse: a meta-analysis of the sinonasal microbiota in chronic rhinosinusitis. Environ Microbiol 2017;19:381-92. [Crossref] [PubMed]
- Gordts F, Halewyck S, Pierard D, et al. Microbiology of the middle meatus: a comparison between normal adults and children. J Laryngol Otol 2000;114:184-8. [Crossref] [PubMed]
- Brożek-Mądry E, Chmielik LP, Gałązka A, et al. Chronic rhinosinusitis in children--bacteriological analysis in terms of cytological examination. Int J Pediatr Otorhinolaryngol 2012;76:512-22. [Crossref] [PubMed]
- Phipps CD, Wood WE, Gibson WS, et al. Gastroesophageal reflux contributing to chronic sinus disease in children: a prospective analysis. Arch Otolaryngol Head Neck Surg 2000;126:831-6. [Crossref] [PubMed]
- Nation J, Kaufman M, Allen M, et al. Incidence of gastroesophageal reflux disease and positive maxillary antral cultures in children with symptoms of chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol 2014;78:218-22. [Crossref] [PubMed]
- Monteiro VR, Sdepanian VL, Weckx L, et al. Twenty-four-hour esophageal pH monitoring in children and adolescents with chronic and/or recurrent rhinosinusitis. Braz J Med Biol Res 2005;38:215-20. [Crossref] [PubMed]
- Sami AS, Scadding GK. Rhinosinusitis in secondary school children-part 2: main project analysis of MSNOT-20 Young Persons Questionnaire (MSYPQ). Rhinology 2014;52:225-30. [Crossref] [PubMed]
- Bhattacharyya N, Jones DT, Hill M, et al. The diagnostic accuracy of computed tomography in pediatric chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg 2004;130:1029-32. [Crossref] [PubMed]
- Al Abduwani J. Cone beam CT paranasal sinuses versus standard multidetector and low dose multidetector CT studies. Am J Otolaryngol 2016;37:59-64. [Crossref] [PubMed]
- Xoran. Available online: https://xorantech.com/RadiationDose/.
- United States Nuclear Regulatory Commission. Naturally occurring background radiation: Backgrounder on Biological Effects of Radiation. 2017.
- Glasier CM, Ascher DP, Williams KD. Incidental paranasal sinus abnormalities on CT of children: clinical correlation. AJNR Am J Neuroradiol 1986;7:861-4. [PubMed]
- Diament MJ, Senac MO Jr, Gilsanz V, et al. Prevalence of incidental paranasal sinuses opacification in pediatric patients: a CT study. J Comput Assist Tomogr 1987;11:426-31. [Crossref] [PubMed]
- Cho BH, Jung YH. Prevalence of incidental paranasal sinus opacification in the dental paediatric patients. Korean Journal of Oral and Maxillofacial Radiology 2008;38:219-23.
- Lesserson JA, Kieserman SP, Finn DG. The radiographic incidence of chronic sinus disease in the pediatric population. Laryngoscope 1994;104:159-66. [Crossref] [PubMed]
- Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology 1993;31:183-4. [PubMed]
- Hill M, Bhattacharyya N, Hall TR, et al. Incidental paranasal sinus imaging abnormalities and the normal Lund score in children. Otolaryngol Head Neck Surg 2004;130:171-5. [Crossref] [PubMed]
- Melder K, Shaffer A, Govil N, et al. The Pediatric Sinus Staging System: A Computed Tomography-Based Approach to Grading Pediatric Sinus Disease. Laryngoscope 2021;131:E642-8. [Crossref] [PubMed]
- Mulligan JK, White DR, Wang EW, et al. Vitamin D3 deficiency increases sinus mucosa dendritic cells in pediatric chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg 2012;147:773-81. [Crossref] [PubMed]
- Elbistanlı MS, Koçak HE, Güneş S, et al. Vit D deficiency is a possible risk factor in ARS. Eur Arch Otorhinolaryngol 2017;274:3391-5. [Crossref] [PubMed]
- Brehm JM, Celedón JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009;179:765-71. [Crossref] [PubMed]
- Ginde AA, Mansbach JM, Camargo CA Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 2009;169:384-90. [Crossref] [PubMed]
- Prescott CA, Prescott KE. Peak nasal inspiratory flow measurement: an investigation in children. Int J Pediatr Otorhinolaryngol 1995;32:137-41. [Crossref] [PubMed]
- Papachristou A, Bourli E, Aivazi D, et al. Normal peak nasal inspiratory flow rate values in Greek children and adolescents. Hippokratia 2008;12:94-7. [PubMed]
- da Cunha Ibiapina C, Ribeiro de Andrade C, Moreira Camargos PA, et al. Reference values for peak nasal inspiratory flow in children and adolescents in Brazil. Rhinology 2011;49:304-8. [Crossref] [PubMed]
- van Spronsen E, Ebbens FA, Fokkens WJ. Normal peak nasal inspiratory flow rate values in healthy children aged 6 to 11 years in the Netherlands. Rhinology 2012;50:22-5. [Crossref] [PubMed]
- Laine-Alava MT, Murtolahti S, Crouse UK, et al. Upper airway resistance during growth: A longitudinal study of children from 8 to 17 years of age. Angle Orthod 2016;86:610-6. [Crossref] [PubMed]
- Kay DJ, Rosenfeld RM. Quality of life for children with persistent sinonasal symptoms. Otolaryngol Head Neck Surg 2003;128:17-26. [Crossref] [PubMed]
- Shapiro GG, Virant FS, Furukawa CT, et al. Immunologic defects in patients with refractory sinusitis. Pediatrics 1991;87:311-6. [PubMed]
- Vanlerberghe L, Joniau S, Jorissen M. The prevalence of humoral immunodeficiency in refractory rhinosinusitis: a retrospective analysis. B-ENT 2006;2:161-6. [PubMed]
- Vichyanond P, Suratannon C, Lertbunnaphong P, et al. Clinical characteristics of children with non-allergic rhinitis vs with allergic rhinitis. Asian Pac J Allergy Immunol 2010;28:270-4. [PubMed]
- Wahn U. The Allergic March. Available online: https://www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/the-allergic-march
- Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol 2001;108:S2-8. [Crossref] [PubMed]
- Sedaghat AR, Phipatanakul W, Cunningham MJ. Prevalence of and associations with allergic rhinitis in children with chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol 2014;78:343-7. [Crossref] [PubMed]
- Brook I, Yocum P, Shah K. Aerobic and anaerobic bacteriology of concurrent chronic otitis media with effusion and chronic sinusitis in children. Arch Otolaryngol Head Neck Surg 2000;126:174-6. [Crossref] [PubMed]
- Fujita A, Honjo I, Kurata K, et al. Refractory otitis media with effusion from viewpoints of eustachian tube dysfunction and nasal sinusitis. Am J Otolaryngol 1993;14:187-90. [Crossref] [PubMed]
- Nickman NJ. Sinusitis, otitis and adenotonsillitis in children: a retrospective study. Laryngoscope 1978;88:117-21. [Crossref] [PubMed]
- Leo G, Piacentini E, Incorvaia C, et al. Prevalence of tympanometric alterations in children with chronic sinusitis. Int J Pediatr Otorhinolaryngol 2008;72:315-9. [Crossref] [PubMed]
- Schenkel EJ, Skoner DP, Bronsky EA, et al. Absence of growth retardation in children with perennial allergic rhinitis after one year of treatment with mometasone furoate aqueous nasal spray. Pediatrics 2000;105:E22 [Crossref] [PubMed]
- Wei JL, Sykes KJ, Johnson P, et al. Safety and efficacy of once-daily nasal irrigation for the treatment of pediatric chronic rhinosinusitis. Laryngoscope 2011;121:1989-2000. [Crossref] [PubMed]
- Pham V, Sykes K, Wei J. Long-term outcome of once daily nasal irrigation for the treatment of pediatric chronic rhinosinusitis. Laryngoscope 2014;124:1000-7. [Crossref] [PubMed]
- Tantry S. Efficacy and safety of GSP 301 nasal spray in children (aged 6 to under 12 years). with seasonal allergic rhinitis (SAR). 2019. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03463031?term=olopatadine&recrs=e&type=Intr&cond=Allergic+Rhinitis&fund=2&draw=3&rank=14
- Brook I. The role of antibiotics in pediatric chronic rhinosinusitis. Laryngoscope Investig Otolaryngol 2017;2:104-8. [Crossref] [PubMed]
- Chandy Z, Ference E, Lee JT. Clinical Guidelines on Chronic Rhinosinusitis in Children. Curr Allergy Asthma Rep 2019;19:14. [Crossref] [PubMed]
- Garbutt JM, Goldstein M, Gellman E, et al. A randomized, placebo-controlled trial of antimicrobial treatment for children with clinically diagnosed acute sinusitis. Pediatrics 2001;107:619-25. [Crossref] [PubMed]
- Brook I, Gober AE. Emergence of beta-lactamase-producing aerobic and anaerobic bacteria in the oropharynx of children following penicillin chemotherapy. Clin Pediatr (Phila) 1984;23:338-41. [Crossref] [PubMed]
- Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 2012;54:e72-e112. [Crossref] [PubMed]
- van Velzen AG, van Riel AJ, Hunault C, et al. A case series of xylometazoline overdose in children. Clin Toxicol (Phila) 2007;45:290-4. [Crossref] [PubMed]
- Eddy O, Howell JM. Are one or two dangerous? Clonidine and topical imidazolines exposure in toddlers. J Emerg Med 2003;25:297-302. [Crossref] [PubMed]
- El-Serag HB, Gilger M, Kuebeler M, et al. Extraesophageal associations of gastroesophageal reflux disease in children without neurologic defects. Gastroenterology 2001;121:1294-9. [Crossref] [PubMed]
- Brietzke SE, Brigger MT. Adenoidectomy outcomes in pediatric rhinosinusitis: a meta-analysis. Int J Pediatr Otorhinolaryngol 2008;72:1541-5. [Crossref] [PubMed]
- Bettadahalli V, Chakravarti A. Post-adenoidectomy quality of life in children with refractory chronic rhinosinusitis. J Laryngol Otol 2017;131:773-8. [Crossref] [PubMed]
- Torretta S, Guastella C, Marchisio P, et al. Sinonasal-Related Orbital Infections in Children: A Clinical and Therapeutic Overview. J Clin Med 2019;8:101. [Crossref] [PubMed]
- Ramadan HH. Adenoidectomy vs endoscopic sinus surgery for the treatment of pediatric sinusitis. Arch Otolaryngol Head Neck Surg 1999;125:1208-11. [Crossref] [PubMed]
- Hayton CH. An investigation into the results of the submucous resection of the septum in children. J Laryngol Otol 1916;132-8. [Crossref]
- Sarnat BG, Wexler MR. Growth of the face and jaws after resection of the septal cartilage in the rabbit. Am J Anat 1966;118:755-67. [Crossref] [PubMed]
- WEXLER MR. SARNAT BG. Rabbit snout growth. Effect of injury to septovomeral region. Arch Otolaryngol 1961;74:305-13. [Crossref] [PubMed]
- Tasca I, Compadretti GC. Nasal growth after pediatric septoplasty at long-term follow-up. Am J Rhinol Allergy 2011;25:e7-12. [Crossref] [PubMed]
- Béjar I, Farkas LG, Messner AH, et al. Nasal growth after external septoplasty in children. Arch Otolaryngol Head Neck Surg 1996;122:816-21. [Crossref] [PubMed]
- El-Hakim H, Crysdale WS, Abdollel M, et al. A study of anthropometric measures before and after external septoplasty in children: a preliminary study. Arch Otolaryngol Head Neck Surg 2001;127:1362-6. [Crossref] [PubMed]
- Lee VS, Gold RM, Parikh SR. Short-term quality of life outcomes following pediatric septoplasty. Acta Otolaryngol 2017;137:293-6. [Crossref] [PubMed]
- Lawrence R. Pediatric septoplasy: a review of the literature. Int J Pediatr Otorhinolaryngol 2012;76:1078-81. [Crossref] [PubMed]
- Manteghi A, Din H, Bundogji N, et al. Pediatric septoplasty and functional septorhinoplasty: A quality of life outcome study. Int J Pediatr Otorhinolaryngol 2018;111:16-20. [Crossref] [PubMed]
- Dispenza F, Saraniti C, Sciandra D, et al. Management of naso-septal deformity in childhood: long-term results. Auris Nasus Larynx 2009;36:665-70. [Crossref] [PubMed]
- GILBERT JG. SEGAL S Jr. Growth of the nose and the septorhinoplastic problem in youth. AMA Arch Otolaryngol 1958;68:673-82. [Crossref] [PubMed]
- Calvo-Henríquez C, Neves JC, Arancibia-Tagle D, et al. Does pediatric septoplasty compromise midfacial growth? A systematic review. Eur Arch Otorhinolaryngol 2020;277:1565-74. [Crossref] [PubMed]
- Grymer LF, Pallisgaard C, Melsen B. The nasal septum in relation to the development of the nasomaxillary complex: a study in identical twins. Laryngoscope 1991;101:863-8. [Crossref] [PubMed]
- Crysdale WS, Walker PJ. External septorhinoplasty in children: patient selection and surgical technique. J Otolaryngol 1994;23:28-31. [PubMed]
- Christophel JJ, Gross CW. Pediatric septoplasty. Otolaryngol Clin North Am 2009;42:287-94. ix. [Crossref] [PubMed]
- Justicz N, Choi S. When Should Pediatric Septoplasty Be Performed for Nasal Airway Obstruction? Laryngoscope 2019;129:1489-90. [Crossref] [PubMed]
- Emami AJ, Brodsky L, Pizzuto M. Neonatal septoplasty: case report and review of the literature. Int J Pediatr Otorhinolaryngol 1996;35:271-5. [Crossref] [PubMed]
- Sooknundun M, Kacker SK, Bhatia R, et al. Nasal septal deviation: effective intervention and long term follow-up. Int J Pediatr Otorhinolaryngol 1986;12:65-72. [Crossref] [PubMed]
- Tasca I, Compadretti GC. Immediate correction of nasal septum dislocation in newborns: long-term results. Am J Rhinol 2004;18:47-51. [Crossref] [PubMed]
- Sorri M, Laitakari K, Vainio-Mattila J, et al. Immediate correction of congenital nasal deformities; follow-up of 8 years. Int J Pediatr Otorhinolaryngol 1990;19:277-83. [Crossref] [PubMed]
- Cingi C, Muluk NB, Ulusoy S, et al. Septoplasty in children. Am J Rhinol Allergy 2016;30:e42-7. [Crossref] [PubMed]
- Barham HP, Thornton MA, Knisely A, et al. Long-term outcomes in medial flap inferior turbinoplasty are superior to submucosal electrocautery and submucosal powered turbinate reduction. Int Forum Allergy Rhinol 2016;6:143-7. [Crossref] [PubMed]
- Arganbright JM, Jensen EL, Mattingly J, et al. Utility of Inferior Turbinoplasty for the Treatment of Nasal Obstruction in Children: A 10-Year Review. JAMA Otolaryngol Head Neck Surg 2015;141:901-4. [Crossref] [PubMed]
- Segal S, Eviatar E, Berenholz L, et al. Inferior turbinectomy in children. Am J Rhinol 2003;17:69-73; discussion 69. [Crossref] [PubMed]
- Ameli F, Brocchetti F, Tosca MA, et al. Adenoidal hypertrophy and allergic rhinitis: is there an inverse relationship? Am J Rhinol Allergy 2013;27:e5-10. [Crossref] [PubMed]
- Ramadan HH. Safety and feasibility of balloon sinuplasty for treatment of chronic rhinosinusitis in children. Ann Otol Rhinol Laryngol 2009;118:161-5. [Crossref] [PubMed]
- Ramadan HH, Bueller H, Hester ST, et al. Sinus balloon catheter dilation after adenoidectomy failure for children with chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg 2012;138:635-7. [Crossref] [PubMed]
- Soler ZM, Rosenbloom JS, Skarada D, et al. Prospective, multicenter evaluation of balloon sinus dilation for treatment of pediatric chronic rhinosinusitis. Int Forum Allergy Rhinol 2017;7:221-9. [Crossref] [PubMed]
- Mirza AA, Shawli HY, Alandejani TA, et al. Efficacy and safety of paranasal sinus balloon catheter dilation in pediatric chronic rhinosinusitis: a systematic review. J Otolaryngol Head Neck Surg 2020;49:69. [Crossref] [PubMed]
- Thottam PJ, Haupert M, Saraiya S, et al. Functional endoscopic sinus surgery (FESS) alone versus balloon catheter sinuplasty (BCS) and ethmoidectomy: a comparative outcome analysis in pediatric chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol 2012;76:1355-60. [Crossref] [PubMed]
- Gerber ME, Kennedy AA. Adenoidectomy With Balloon Catheter Sinuplasty: A Randomized Trial for Pediatric Rhinosinusitis. Laryngoscope 2018;128:2893-7. [Crossref] [PubMed]
- Younis RT, Lazar RH. Criteria for success in pediatric functional endonasal sinus surgery. Laryngoscope 1996;106:869-73. [Crossref] [PubMed]
- Vlastarakos PV, Fetta M, Segas JV, et al. Functional endoscopic sinus surgery improves sinus-related symptoms and quality of life in children with chronic rhinosinusitis: a systematic analysis and meta-analysis of published interventional studies. Clin Pediatr (Phila) 2013;52:1091-7. [Crossref] [PubMed]
- Hebert RL 2nd, Bent JP 3rd. Meta-analysis of outcomes of pediatric functional endoscopic sinus surgery. Laryngoscope 1998;108:796-9. [Crossref] [PubMed]
- Makary CA, Ramadan HH. The role of sinus surgery in children. Laryngoscope 2013;123:1348-52. [Crossref] [PubMed]
- Rudnick EF, Mitchell RB. Long-term improvements in quality-of-life after surgical therapy for pediatric sinonasal disease. Otolaryngol Head Neck Surg 2007;137:873-7. [Crossref] [PubMed]
- Alekseenko S, Karpischenko S, Artyushkin S, et al. Ciliary function and sinonasal mucosal cytology in pediatric patients with chronic rhinosinusitis during a year after functional endoscopic sinus surgery. Rhinology 2021;59:319-27. [PubMed]
- Fetta M, Tsilis NS, Segas JV, et al. Functional endoscopic sinus surgery improves the quality of life in children suffering from chronic rhinosinusitis with nasal polyps. Int J Pediatr Otorhinolaryngol 2017;100:145-8. [Crossref] [PubMed]
- Siedek V, Stelter K, Betz CS, et al. Functional endoscopic sinus surgery--a retrospective analysis of 115 children and adolescents with chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol 2009;73:741-5. [Crossref] [PubMed]
- Bothwell MR, Piccirillo JF, Lusk RP, et al. Long-term outcome of facial growth after functional endoscopic sinus surgery. Otolaryngol Head Neck Surg 2002;126:628-34. [Crossref] [PubMed]
- Senior B, Wirtschafter A, Mai C, et al. Quantitative impact of pediatric sinus surgery on facial growth. Laryngoscope 2000;110:1866-70. [Crossref] [PubMed]
- Chen W, Gardner PA, Branstetter BF, et al. Long-term impact of pediatric endoscopic endonasal skull base surgery on midface growth. J Neurosurg Pediatr 2019;23:523-30. [Crossref] [PubMed]
- Wu PW, Huang CC, Yang SW, et al. Endoscopic sinus surgery for pediatric patients: Prognostic factors related to revision surgery. Laryngoscope 2020;130:1051-5. [Crossref] [PubMed]
- Meltzer NE, Tunkel DE. Outcome of Endoscopic Sinus Surgery in Children With Allergic Rhinitis. Pediatrics 2007;120:S126. [Crossref]
- Manning SC, Wasserman RL, Silver R, et al. Results of endoscopic sinus surgery in pediatric patients with chronic sinusitis and asthma. Arch Otolaryngol Head Neck Surg 1994;120:1142-5. [Crossref] [PubMed]
- Khalfoun S, Tumin D, Ghossein M, et al. Improved Lung Function after Sinus Surgery in Cystic Fibrosis Patients with Moderate Obstruction. Otolaryngol Head Neck Surg 2018;158:381-5. [Crossref] [PubMed]
- Roxbury CR, Li L, Rhee D, et al. Safety and Perioperative Adverse Events in Pediatric Endoscopic Sinus Surgery: An ACS-NSQIP-P Analysis. Int Forum Allergy Rhinol 2017;7:827-36. [Crossref] [PubMed]
- McKeon M, Medina G, Kawai K, et al. Readmissions following ambulatory pediatric endoscopic sinus surgery. Laryngoscope 2019;129:2681-6. [Crossref] [PubMed]
- Stern-Shavit S, Nachalon Y, Leshno M, et al. Middle meatal packing in endoscopic sinus surgery-to pack or not to pack?-a decision-analysis model. Laryngoscope 2017;127:1506-12. [Crossref] [PubMed]
- Orlandi RR, Lanza DC. Is nasal packing necessary following endoscopic sinus surgery? Laryngoscope 2004;114:1541-4. [Crossref] [PubMed]
- Hobson CE, Choby GW, Wang EW, et al. Systematic review and metaanalysis of middle meatal packing after endoscopic sinus surgery. Am J Rhinol Allergy 2015;29:135-40. [Crossref] [PubMed]
- Alsaffar H, Sowerby L, Rotenberg BW. Postoperative nasal debridement after endoscopic sinus surgery: a randomized controlled trial. Ann Otol Rhinol Laryngol 2013;122:642-7. [Crossref] [PubMed]
- Kemppainen T, Seppä J, Tuomilehto H, et al. Repeated early debridement does not provide significant symptomatic benefit after ESS. Rhinology 2008;46:238-42. [PubMed]
- Lee JY, Byun JY. Relationship between the frequency of postoperative debridement and patient discomfort, healing period, surgical outcomes, and compliance after endoscopic sinus surgery. Laryngoscope 2008;118:1868-72. [Crossref] [PubMed]
- Varsak YK, Yuca K, Eryılmaz MA, et al. Single seventh day debridement compared to frequent debridement after endoscopic sinus surgery: a randomized controlled trial. Eur Arch Otorhinolaryngol 2016;273:689-95. [Crossref] [PubMed]
- Tzelnick S, Alkan U, Leshno M, et al. Sinonasal debridement versus no debridement for the postoperative care of patients undergoing endoscopic sinus surgery. Cochrane Database Syst Rev 2018;11:CD011988 [Crossref] [PubMed]
- Helmen ZM, Little RE, Robey T. Utility of Second-Look Endoscopy with Debridement After Pediatric Functional Endoscopic Sinus Surgery in Patients with Cystic Fibrosis. Ann Otol Rhinol Laryngol 2020;129:1153-62. [Crossref] [PubMed]
- Glatz P, Sandin RH, Pedersen NL, et al. Association of Anesthesia and Surgery During Childhood With Long-term Academic Performance. JAMA Pediatr 2017;171:e163470 [Crossref] [PubMed]
- Walkden GJ, Gill H, Davies NM, et al. Early Childhood General Anesthesia and Neurodevelopmental Outcomes in the Avon Longitudinal Study of Parents and Children Birth Cohort. Anesthesiology 2020;133:1007-20. [Crossref] [PubMed]
Cite this article as: Campbell RG. Paediatric sinonasal surgery: a literature review. Aust J Otolaryngol 2021;4:31.