
Endoscopic management of en-plaque cholesteatoma associated with tympanic membrane perforations
Introduction
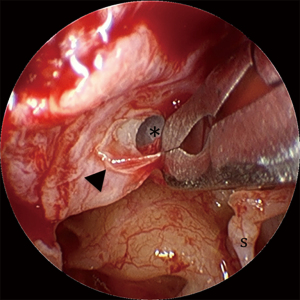
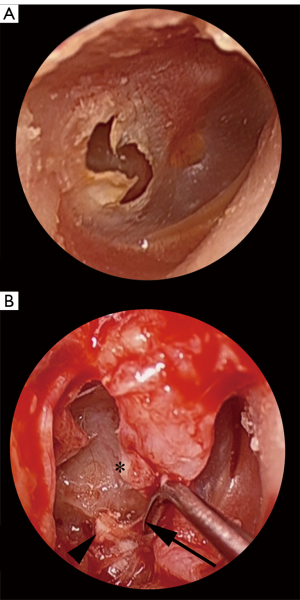
Cholesteatoma associated with tympanic membrane (TM) perforations often forms as a sheet-like distribution of epithelial cells on the medial surface of the TM (Figure 1). Due to this unique arrangement, the authors of this study refer to this entity as ‘en-plaque cholesteatoma (EPC)’, referring to the in-growth of squamous epithelium onto the under surface of the TM by more than 2 mm. This distance is used as it represents more than the usual margin removed when freshening the squamomucosal junction in preparation for grafting. Un-treated EPC can progress to extensive mesotympanic and middle ear disease that can lead to significant morbidity (Figure 2A,2B) (1). The term ‘en-plaque’ was featured previously in the literature in relation to meningioma and is employed when the growth of the tumour occurs in a sheet-like fashion along the dura, analogous to the flat, plaque-like, growth of cholesteatoma on the medial surface of the TM (2,3).
Marginal perforations of the TM are classically associated with the formation of cholesteatoma. This has traditionally made their diagnosis, monitoring and management distinct from central perforations. However, central perforations can also lead to the formation of cholesteatoma and so, importantly, should also be treated with the same caution and index of suspicion as marginal or attic perforations (4-7). The postulated theory for cholesteatoma formation in the setting of perforations suggests that migrating squamous epithelium in the external canal arises from stem cells in the umbo and annulus. Disturbance of the migration pathway at the edge of a perforation, from either trauma or infection, results in epithelium growing medially on to the medial surface of the TM and into the middle ear cavity (8,9).
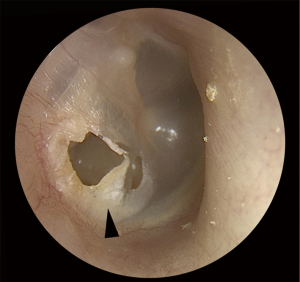
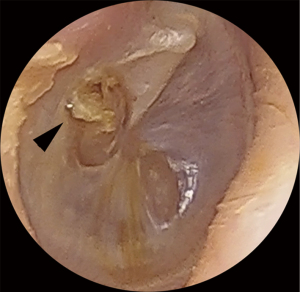
EPC may be difficult to identify on examination. Classic clinical findings include granulation (Figure 3) or irregular epithelium at the margin of the perforation (Figure 4). Other clinical clues can include a conductive hearing loss disproportionate to the size of the perforation. CT scans may not be able to distinguish between a thickened TM, granulation tissue, otorrhea and cholesteatoma. Non-echo-planar imaging diffusion-weighted imaging magnetic resonance imaging (non-EPI DWI MRI) is frequently falsely negative due the small volume and spreading nature of disease (10,11). Additionally, failure to identify disease arising from a margin of a perforation may result in attempted tympanoplasty, only to intraoperatively discover the presence of occult cholesteatoma, necessitating the need for more extensive and unforeseen surgery. If the disease is missed intraoperatively then implantation cholesteatoma or graft failure may occur (12).
Traditionally, the operative approach to cholesteatoma management has been with the operating microscope. In recent years endoscopic approaches to the ear provide a supplement or alternative to traditional microscopic methods, with similar clearance and recurrence rates (13). Straight and angled endoscopes scopes, via a transcanal approach, have been shown to improve visualisation, not only of perforations, but also of most anatomical regions and key recesses that may be involved with EPC (14-24). Utilising endoscopes in the clinical assessment and operative treatment of TM perforations may provide a transcanal alternative to traditional microscopic techniques. Additionally, transcanal endoscopic ear approaches have been shown to be safe and be associated with other benefits, including reduced post-operative pain and shorter post-operative hospital stays when compared to microscopic post-auricular approaches (25-27). The aim of this study is to highlight EPC as an under recognised entity and report the first published outcomes of its management via endoscopic trans-canal techniques.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/ajo-20-86).
Methods
A retrospective chart audit of all patients with EPC treated by four senior surgeons between 2012 and 2018 was undertaken. EPC was defined as cholesteatoma associated with the margin of a TM perforation and identified based on the intra-operative findings of the surgeon. Cases were required to have a primary diagnosis of cholesteatoma, excluding revision cases. Cases where the patient had undergone a previous myringoplasty were excluded. Only cases involving endoscopic ear surgery techniques (Massachusetts Class 2A/B or 3) were included.
Patient medical records and surgical correspondence were used to obtain demographic, follow up and outcome data. Pre and postoperative Pure Tone Audiometry (PTA) results were obtained by averaging results for air conduction at 0.5, 1, 2 and 3 kHz. Operative reports and case records were utilized to obtain operative data including the surgical approach, microscopic view, endoscope usage, extent of disease and the graft material used for tympanoplasty. Endoscope usage was graded using the grading system proposed by Cohen et.al. (28)
Statistical analysis
Data from all 4 surgeons was collated and utilisng Microsoft ExcelTM, descriptive statistics were used to define the cohort. Paired t-tests were performed for comparison of preoperative and postoperative PTA means.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the North Sydney Local Health District Human Research Ethics Committee - HREC reference: LNR/18/HAWKE/38. Individual consent for this retrospective analysis was waived.
Results
A total of 37 patients met the criteria for inclusion in the study, 25 (67.6%) were male and 12 (32.4%) of the patients were female. The average age of the cohort was 46 (range, 12–78) years. Seven (18.9%) patients were known to have diabetes mellitus, six (16.2%) patients were immunosuppressed for other reasons and eight patients (21.6%) had a prior history of smoking.
The perforation was right sided in 23 (62.2%) patients; central in 10 (27%) cases and marginal in 27 (73%) cases. Where the perforation was marginal the most common site was in the posterior portion of the TM, 19 (51.4%) patients, followed by inferior in six (16.2%) patients and anterior in two (5.4%) patients. The size of the perforation varied between patients with a range of 5–60% of the total area of the TM.
All patients underwent underlay tympanoplasty as part of the procedure with a fascia graft being utilized in six (16.2%) cases and composite cartilage graft in 31 (83.8%). Persistence of the perforation following tympanoplasty was noted in 4 cases, giving an overall closure rate of 89.2%. All the patients that experienced graft failure were noted to have a marginal perforation. Statistical analysis of predisposing patient factors with respect to TM re-perforation was not possible given the few cases of graft failure. Furthermore, inspection of the four cases of graft failure identified no consistent risk factor.
Cholesteatoma extent was limited to the mesotympanum in 35 (94.6%) of cases with the retrotympanum and hypotympanum involved in a one (2.7%) case each. In five (13.5%) cases the disease involved the stapes footplate and the round window was affected in nine (24.3%) cases. Ossicular involvement was noted in 11 (29.7%) cases, necessitating removal of part of the ossicular chain. A total endoscopic approach (Class 3) was possible in 27 cases (73%). Of the remaining cases, five required significant microscopic assistance (Class 2A, 13.5%) and four needed minimal microscopic involvement (Class 2B, 10.8%). All cases were completed transcanal with only three (8.1%) patients requiring a canaloplasty and 11 (29.7%) requiring an endoscopic atticotomy for operative access. The microscope was employed when two hands were required including to provide counter traction when excising involved TM or with bleeding around the stapes.
Primary ossicular chain reconstruction was performed in seven (18.9%) cases: two (5.4%) patients had a total ossicular replacement prosthesis (TORP), four (10.8%) patients had a partial ossicular replacement prosthesis (PORP) and one patient (2.7%) had a cartilage interposition graft. Complete PTA data was available for 6 of the 7 cases that underwent OCR, there was no statistically significant difference between pre- vs. postoperative PTA in this subgroup (37.7 vs. 33.0 dB, P=0.21). Additionally, complete PTA data was available for 32 of the 37 cases in the cohort, similarly, there was no statistically significant difference between pre- vs. postoperative PTA (32.2 vs. 30.7 dB, P=0.38) (Figures 5,6). There were no significant intraoperative or postoperative complications recorded.
The mean follow-up interval was 22 (range, 6–66) months. Three (8.1%) patients were lost to follow up. Residual cholesteatoma disease was noted in two (5.4%) patients. In the two patients with residual disease, one presented with a 10% posterior perforation and the other had a 50% anterior perforation. No meaningful statistical analysis could be conducted, given the low rate of residual disease.
Discussion
To date, there are no studies addressing the endoscopic management of EPC associated with perforations. This study highlights the importance of routine and thorough inspection of the TM both pre and intraoperatively for the presence of cholesteatoma associated with the margin of a perforation prior to tympanoplasty. The endoscope is particularly well suited to this application and can be a useful adjunct to the microscope if there is any doubt. Additionally, this study also demonstrates that endoscopic management of EPC is safe, feasible, and is associated with low levels of residual disease or residual perforation. The majority of patients (73%) in this study underwent a totally endoscopic approach with minimal need for surgical optimization to achieve an acceptable endoscopic view (i.e., atticotomy or canaloplasty). While the assessment and management of persistent perforations has historically utilized microscopy, the use of endoscopes for the assessment and treatment of otologic disease and conditions is becoming more common and proving to have several benefits. Among these benefits are those ascribed to minimally invasive surgery in general, including reduced trauma to healthy tissue and a decrease in post-operative pain, shorter periods of hospitalization and reduced complication rates (25-27).
Marginal perforations are associated with a higher risk of developing secondary acquired cholesteatoma. Importantly, despite being thought of as ‘safe’ perforations, central perforations can also be associated with the development of EPC. In one study, as many as 13.7% of cholesteatomas were associated with a marginal perforation and a further 13.3% were associated with central perforations (4). Similar results were noted in the present study with a rate of EPC of 7.6% during the study period, highlighting the importance of routinely excluding pathology prior to myringoplasty.
Despite only three of the cases presented in this series having anterior perforations, specific difficulties with the microscopic visualization of anterior perforations have been reported. Ayache et al. (19) and Tseng et al. (20) reported incomplete visualization of an anterior marginal perforation using microscopy and otoscopy in 73% and 37% respectively. The ability of the endoscope to visualize the extent of the perforation, guide graft placement and inspect the medial aspect of the perforation to identify EPC, may be most beneficial for anterior perforations.
Rates of graft success following microscopic surgery have been reported to be as high as 90 to 95% in the literature, with similar rates of 80% to 100% success being reported by authors employing an endoscopic technique (16,29-33). Lade et al. (17) and Harugop et al. (15) compared both techniques and found no difference in success rates. In the case of anterior perforations, where microscopic visualization can be difficult, success rates of 88% to 98% have been reported using a microscope, with Tseng et al. (20) reporting a similar success rate of 93% using an endoscopic approach. The results from this work show a graft success rate of 89.2%, comparable to the findings of non-cholesteatoma perforation series, suggesting the presence of the cholesteatoma in itself does not impact on the success of the subsequent reconstruction, provided it is correctly identified and appropriately extirpated.
Aside from the enhanced ability to visualize the lateral surface of the TM and associated marginal perforations, the use of angled endoscopes also facilitates the inspection of the medial surface of the TM. This allows for the detection of epithelial inclusions and occult cholesteatoma where microscopy and non-EPI DWI MRI can be falsely reassuring prior to surgery (10,11). Additionally, it allows for the identification of the muco-epithelial junction and facilitates the removal of squamous epithelium that has migrated onto the medial surface.
Somers et al. (34) performed a histological study of whole TM’s with central perforations and found that the mucoepithelial junction was not at the rim of the perforation but on the medial surface of the membrane in 30% of cases. The extent of squamous migration onto the medial surface of the TM ranged from 0.2 to 1.15 mm from the rim of the perforation and in 7% of cases, squamous epithelial inclusions were noted, involving both the TM and ossicular chain. Similarly, Yadav et al. (32) also found that the mucoepithelial junction resided on the under surface of the TM in 30% of cases and emphasized the importance of examining the medial surface of the TM with an endoscope to prevent the formation of iatrogenic cholesteatoma. These observations highlight the importance of thorough examination of the medial aspect of the TM in all perforations. The endoscopic approach facilitates this well and should be considered even in cases performed predominantly with the microscope.
The optical advantage provided by the endoscope, translates to improved assessment of the middle ear cleft, the medial aspect of the TM and any associated perforation, reducing the chance of leaving residual cholesteatoma in areas difficult to visualize with the microscope alone. Cholesteatoma in this series was limited to the mesotympanum in the majority of cases, resulting in complete removal with a totally endoscopic approach. In the cases with more extensive disease, the microscope will be a crucial additional asset for access. While primary endoscopic approach is favored by the authors whenever possible, the operating microscopic remains indispensable in situations with extensive disease requiring traditional open approaches.
The findings of this study need to be considered in the context of two main limitations. Firstly, the cohort examined had a relatively short follow up period, potentially underestimating the true rate of residual or recurrent disease in this cohort. However, the rates reported in this series are in keeping with the findings of other authors reporting their experience with the endoscopic management of cholesteatoma. Secondly, the relatively low number of cases over a 7-year period across 4 surgeons may introduce a level of bias due to inter-operative variability in surgical approach.
Conclusions
This retrospective case series constitutes the first appraisal of the endoscopic management of EPC associated with the margin of a TM perforation. This entity should be routinely considered when faced with a patient presenting with persistent TM perforation and the presence of cholesteatoma actively looked for prior to intervention. The endoscopic approach presents a safe and effective technique for the eradication of disease limited to the middle ear and can be combined with conventional microscopic surgical techniques with success when more extensive disease is present. The rates of perforation closure and eradication of cholesteatoma are comparable to other published series.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/ajo-20-86
Data Sharing Statement: Available at https://dx.doi.org/10.21037/ajo-20-86
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/ajo-20-86). NJ and AJS serve as unpaid editorial board members of the Australian Journal of Otolaryngology from Jan 2019 to Dec 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the North Sydney Local Health District Human Research Ethics Committee - HREC reference: LNR/18/HAWKE/38 and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yung M, Tono T, Olszewska E, et al. EAONO/JOS Joint Consensus Statements on the Definitions, Classification and Staging of Middle Ear Cholesteatoma. J Int Adv Otol 2017;13:1-8. [Crossref] [PubMed]
- De Jesús O, Toledo MM. Surgical management of meningioma en plaque of the sphenoid ridge. Surg Neurol 2001;55:265-9. [Crossref] [PubMed]
- Basu K, Majumdar K, Chatterjee U, et al. En plaque meningioma with angioinvasion. Indian J Pathol Microbiol 2010;53:319-21. [Crossref] [PubMed]
- Aquino JE, Cruz Filho NA, de Aquino JN. Epidemiology of middle ear and mastoid cholesteatomas: study of 1146 cases. Braz J Otorhinolaryngol 2011;77:341-7. [Crossref] [PubMed]
- Rout M, Mohanty D, Vijaylaxmi Y, et al. Prevalence of cholesteatoma in chronic suppurative otitis media with central perforation. Indian J Otol 2012;18:7. [Crossref]
- Oktay MF, Cureoglu S, Schachern PA, et al. Tympanic membrane changes in central tympanic membrane perforations. Am J Otolaryngol 2005;26:393-7. [Crossref] [PubMed]
- Rice DH. Central perforations-safe or unsafe? Arch Otolaryngol 1975;101:626-7. [Crossref] [PubMed]
- Louw L. Acquired cholesteatoma pathogenesis: stepwise explanations. J Laryngol Otol 2010;124:587-93. [Crossref] [PubMed]
- Jackler RK, Santa Maria PL, Varsak YK, et al. A new theory on the pathogenesis of acquired cholesteatoma: Mucosal traction. Laryngoscope 2015;125:S1-S14. [Crossref] [PubMed]
- Jindal M, Riskalla A, Jiang D, et al. A systematic review of diffusion-weighted magnetic resonance imaging in the assessment of postoperative cholesteatoma. Otol Neurotol 2011;32:1243-9. [Crossref] [PubMed]
- Khemani S, Singh A, Lingam RK, et al. Imaging of postoperative middle ear cholesteatoma. Clin Radiol 2011;66:760-7. [Crossref] [PubMed]
- Nejadkazem M, Totonchi J, Naderpour M, et al. Intratympanic membrane cholesteatoma after tympanoplasty with the underlay technique. Arch Otolaryngol Head Neck Surg 2008;134:501-2. [Crossref] [PubMed]
- Presutti L, Gioacchini FM, Alicandri-Ciufelli M, et al. Results of endoscopic middle ear surgery for cholesteatoma treatment: a systematic review. Acta Otorhinolaryngol Ital 2014;34:153-7. [PubMed]
- Tarabichi M, Kapadia M. Transcanal endoscopic management of acquired Cholesteatoma. Oper Tech Otolayngol Head Neck Surg 2017;28:29-35. [Crossref]
- Harugop AS, Mudhol RS, Godhi RA. A comparative study of endoscope assisted myringoplasty and micrsoscope assisted myringoplasty. Indian J Otolaryngol Head Neck Surg 2008;60:298-302. [Crossref] [PubMed]
- Furukawa T, Watanabe T, Ito T, et al. Feasibility and advantages of transcanal endoscopic myringoplasty. Otol Neurotol 2014;35:e140-5. [Crossref] [PubMed]
- Lade H, Choudhary SR, Vashishth A. Endoscopic vs microscopic myringoplasty: a different perspective. Eur Arch Otorhinolaryngol 2014;271:1897-902. [Crossref] [PubMed]
- Lakpathi G, Sudarshan Reddy L. Anand. Comparative Study of Endoscope Assisted Myringoplasty and Microscopic Myringoplasty. Indian J Otolaryngol Head Neck Surg 2016;68:185-90. [Crossref] [PubMed]
- Ayache S. Cartilaginous myringoplasty: the endoscopic transcanal procedure. Eur Arch Otorhinolaryngol 2013;270:853-60. [Crossref] [PubMed]
- Tseng CC, Lai MT, Wu CC, et al. Endoscopic Transcanal Myringoplasty for Anterior Perforations of the Tympanic Membrane. JAMA Otolaryngol Head Neck Surg 2016;142:1088-93. [Crossref] [PubMed]
- Karchier EB, Niemczyk K, Orłowski A. Comparison of visualization of the middle ear by microscope and endoscopes of 30° and 45° through posterior tympanotomy. Wideochir Inne Tech Maloinwazyjne 2014;9:276-81. [Crossref] [PubMed]
- Presutti L, Marchioni D, Mattioli F, et al. Endoscopic management of acquired cholesteatoma: our experience. J Otolaryngol Head Neck Surg 2008;37:481-7. [PubMed]
- Badr-el-Dine M. Value of ear endoscopy in cholesteatoma surgery. Otol Neurotol 2002;23:631-5. [Crossref] [PubMed]
- Ayache S, Tramier B, Strunski V. Otoendoscopy in cholesteatoma surgery of the middle ear: what benefits can be expected? Otol Neurotol 2008;29:1085-90. [Crossref] [PubMed]
- Kakehata S, Furukawa T, Ito T, et al. Comparison of Postoperative Pain in Patients Following Transcanal Endoscopic Versus Microscopic Ear Surgery. Otol Neurotol 2018;39:847-53. [Crossref] [PubMed]
- Marchioni D, Rubini A, Gazzini L, et al. Complications in Endoscopic Ear Surgery. Otol Neurotol 2018;39:1012-7. [Crossref] [PubMed]
- Glikson E, Yousovich R, Mansour J, et al. Transcanal Endoscopic Ear Surgery for Middle Ear Cholesteatoma. Otol Neurotol 2017;38:e41-5. [Crossref] [PubMed]
- Cohen MS, Basonbul RA, Barber SR, et al. Development and validation of an endoscopic ear surgery classification system. Laryngoscope 2018;128:967-70. [Crossref] [PubMed]
- Sheehy JL, Anderson RG. Myringoplasty. A review of 472 cases. Ann Otol Rhinol Laryngol 1980;89:331-4. [Crossref] [PubMed]
- Rizer FM. Overlay versus underlay tympanoplasty. Part II: the study. Laryngoscope 1997;107:26-36. [Crossref] [PubMed]
- el-Guindy A. Endoscopic transcanal myringoplasty. J Laryngol Otol 1992;106:493-5. [Crossref] [PubMed]
- Yadav SP, Aggarwal N, Julaha M, et al. Endoscope-assisted myringoplasty. Singapore Med J 2009;50:510-2. [PubMed]
- Karhuketo TS, Ilomäki JH, Puhakka HJ. Tympanoscope-assisted myringoplasty. ORL J Otorhinolaryngol Relat Spec 2001;63:353-7; discussion 358. [Crossref] [PubMed]
- Somers Th, Houben V, Goovaerts G, et al. Histology of the perforated tympanic membrane and its muco-epithelial junction. Clin Otolaryngol Allied Sci 1997;22:162-6. [Crossref] [PubMed]
Cite this article as: Rao A, Mooney CP, Ma A, Jufas N, Saxby AJ, Kong J, Patel N. Endoscopic management of en-plaque cholesteatoma associated with tympanic membrane perforations. Aust J Otolaryngol 2021;4:35.




