
Otolaryngology driven percutaneous endoscopic placement of gastrostomy tubes as part of integrative head and neck cancer service
Introduction
Head and neck cancer patients are a complex cohort requiring careful assessment and diagnosis, an often lengthy treatment course, and require a multidisciplinary approach to management. Otolaryngologists are typically involved in diagnosis, management and long-term oncologic surveillance for this patient group. Their anaesthesia and intubation often have increased complexity with distorted anatomy and friable tumours. Nutrition can be suboptimal and may need to be supported prior, during, or following treatment. Head and neck cancer management can include the use of percutaneous endoscopic gastrostomy (PEG) tubes as a medium to long term method of nutritional support (1).
The insertion of a PEG tube has traditionally been competently undertaken by general surgeons and gastroenterologists. The insertion of PEG tubes by otolaryngologists-head and neck (ORL-HN) surgeons for head and neck cancer patients, in North America and Europe, has had similar success and equivalent failure rates, morbidity, and mortality (2,3). Advantageously, an otolaryngology driven PEG service may be associated with fewer patient appointments, logistical and financial efficiencies, and decreased treatment delays than traditional approaches (2,3).
The aim of this study is to describe the effectiveness of an otolaryngology driven PEG placement and to review the associated morbidity in an Australian head and neck cancer centre. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/ajo-21-33).
Methods
Population
A retrospective case series was performed on head and neck cancer patients undergoing cancer therapy, who needed a PEG tube placed as part of their overall management. Decision for PEG placement and subsequent care was undertaken by a multidisciplinary team which included a stoma care nurse and dietician. Patient were consecutively recruited from the St. Vincent’s Head & Neck Cancer Centre from April 2016 to February 2021 with a PEG tube placed by an otolaryngologist. Demographic data, tumour site, stage (AJCC 8th edition), treatment (chemoradiotherapy/surgery), and previous history of PEG tube were collected (4). The indication for PEG insertion was considered ‘reactive’ if the PEG tube was inserted after a clinical need for feeding was identified, and ‘prophylactic’ if the tube was inserted in anticipation that nutritional requirements would not be met during the treatment process. A concurrent procedure was defined as any procedure that was undertaken during the same anaesthetic as the PEG tube insertion.
Exclusion criteria for insertion of PEG tube included: coagulation disorders [international normalized ratio (INR) >1.5, partial thromboplastin time (PTT) >50 s, platelets <50,000/mm3], hemodynamic instability, severe ascites, peritonitis, abdominal wall infection at the selected site of placement, marked peritoneal carcinomatosis, interposed organs (e.g., liver, colon), history of previous laparotomy, history of total gastrectomy, gastric outlet obstruction, severe gastroparesis, and lack of informed consent for the procedure.
Intervention
Gastrostomy tube placement (20 French, MIC percutaneous endoscopic gastrostomy kit, Halyard, GA, USA) was by percutaneous approach with pull-through technique (5). After insertion of the PEG tube a flexible endoscopy was typically undertaken to confirm the position of the PEG bumper unless re-instrumentation was not feasible. All procedures were performed under general anesthesia by a single ORL-HN surgeon, fellowship trained and accredited for PEG insertion. They were assisted by either an otolaryngology surgeon or otolaryngology trainee.
Descriptive of outcomes
Primary outcomes were correct placement of tube and successful function of PEG for enteral nutrition. Time to insertion was recorded as the time between when a patient was identified as requiring a PEG and ready for care; and when the PEG tube was inserted. As a subset within the prophylactic group, if the PEG was inserted during their definitive surgical therapy, this was recorded as zero days. In the reactive group it was the time from decision by the multidisciplinary team for the need to place PEG, to the time of insertion. Whether the placement of PEG was concurrent with an additional procedure was also recorded. Secondary outcomes assessed the 90-day morbidity and procedure related mortality. Morbidity was characterised as major and minor complications based on criteria used by Schapiro and Edmundowicz, and Bannister (6,7). Major complications included: peritonitis, sepsis, intra-abdominal abscess, gastric haemorrhage, intestinal fistula, obstruction at gastric outlet, necrotising fasciitis, buried bumper syndrome, solid organ injury/visceral perforation, PEG site metastasis, and early extrusion of PEG tube. Minor complications included: cellulitis, late extrusion of PEG tube, paralytic ileus, impacted tube lumen, peristomal leakage and haematoma (6,7).
Statistical analysis
All continuous data was treated as parametric and reported as mean ± standard deviation (SD). SPSS version 28.0 statistical software (IBM Corp., Armonk, NY, USA) was used for statistical analysis of data. Statistical significance was determined using independent t-test for continuous data and Pearson’s chi-squared test categorical data. P<0.05 was considered statistically significant.
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Adequate protection of patient privacy and confidentiality was ensured through deidentification and appropriate data storage. Consent was justifiably waived. Research ethics board approval was granted for the study protocol by St. Vincent’s Hospital Human Research Ethics Committee (PID01240).
Results
Population
Ninety-six (n=96) patients were assessed (63.1±11.9 years, 15% female) (Table 1). The weight was 73.8±17.3 kg and the body mass index (BMI) was 24.0±5.1 kg/m2. The most common site of the primary tumour was the oropharynx with 38 patients (40%), hypopharynx 16 (17%), and oral cavity 13 (14%) (Table 1). Tumour stage (T classification) was most common at T3 with 39 (41%) patients, T2 with 26 (27%), and T4 with 15 (16%) patients. Sixty-seven (70%) had PEG placement in the setting of primary cancer care, 26 (27%) PEG placement as part of recurrent cancer and 3 (3%) were in remission but required ongoing nutritional support needs. Of those receiving treatment the primary modality was surgery in 67 patients (69%) and radiotherapy ± chemotherapy for 26 patients (27%). Two patients (2%) received chemotherapy alone.
Table 1
| Characteristics | Total | Uneventful | Complication | P value |
|---|---|---|---|---|
| N | 96 | 87 | 9 | |
| Age (years), mean ± SD | 63.1±11.9 | 65.6±11.8 | 65.4±14.3 | 0.97 |
| Gender, % | 0.45 | |||
| Female | 15 | 14 | 22 | |
| Weight (kg), mean ± SD | 73.8±17.3 | 73.1±17.2 | 80.7±18.8 | 0.27 |
| BMI (kg/m2), mean ± SD | 24.0±5/1 | 23.8±5.0 | 26.8±5.1 | 0.13 |
| Tumour site, % | 0.31 | |||
| Oral cavity | 14 | 15 | 0 | |
| Oropharynx | 40 | 39 | 44 | |
| Hypopharynx | 17 | 17 | 11 | |
| Supraglottis | 9 | 9 | 11 | |
| Larynx | 7 | 8 | 0 | |
| Cutaneous | 3 | 2 | 11 | |
| Nasal/nasopharynx/skull base | 5 | 3 | 22 | |
| Unknown primary | 1 | 1 | 0 | |
| Dysfunctional larynx | 3 | 3 | 0 | |
| Parapharyngeal space | 1 | 1 | 0 | |
| T stage, % | 0.76 | |||
| Tx | 2 | 2 | 0 | |
| T1 | 12 | 10 | 11 | |
| T2 | 27 | 25 | 44 | |
| T3 | 41 | 43 | 22 | |
| T4 | 16 | 16 | 22 | |
| Remission | 3 | 3 | 0 | |
| Disease status, % | 0.78 | |||
| Primary disease | 70 | 69 | 78 | |
| Recurrent disease | 27 | 28 | 22 | |
| Remission | 3 | 3 | 0 | |
| Treatment at insertion, % | 0.89 | |||
| Surgery | 69 | 68 | 78 | |
| Radiation ± chemotherapy | 27 | 28 | 22 | |
| No treatment/remission | 2 | 2 | 0 | |
| Chemotherapy only | 2 | 2 | 0 | |
| PEG history, % | 0.34 | |||
| Primary | 93 | 91 | 100 | |
| Revision | 8 | 9 | 0 | |
| Purpose for insertion, % | 0.48 | |||
| Prophylactic insertion | 40 | 38 | 56 | |
| Reactive insertion | 65 | 62 | 44 |
PEG, percutaneous endoscopic gastrostomy; SD, standard deviation; BMI, body mass index.
PEG tube placement was reactive in 58 (60%) of patients. Thirty-eight (40%) PEG tubes were placed prophylactically. Primary insertions of PEG tubes occurred in 88 patients (92%).
Primary outcome: PEG placement and use
Successful and correct placement of PEG tube occurred in 100% (n=96). All PEG tubes functioned correctly permitting use. Time to placement was 2.2±2.6 days. Thirty-seven (39%) of all PEG insertions were performed with a concurrent procedure. Procedures were varied and ranged from definitive cancer surgery to tumour biopsy and dental extraction (see Table 2).
Table 2
| Procedures | N |
|---|---|
| Definitive surgery (tumour excision ± tracheostomy ± dental extraction ± neck dissection ± reconstruction) | 10 |
| Microlaryngoscopy ± oesophagoscopy ± biopsy ± dental extraction | 10 |
| Neck dissection ± microlaryngoscopy | 5 |
| Oesophageal dilation | 4 |
| Tracheostomy | 2 |
| Montgomery tube + dental extraction | 1 |
| Microlaryngoscopy + re-excision tumour margin | 1 |
| Vocal cord injection + gold weight insertion eyelid | 1 |
| Wound washout | 1 |
| Nasendoscopy + biopsy | 1 |
| Fistula repair | 1 |
PEG, percutaneous endoscopic gastrostomy.
Secondary outcome measures
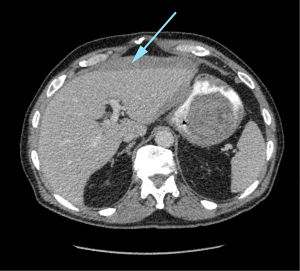
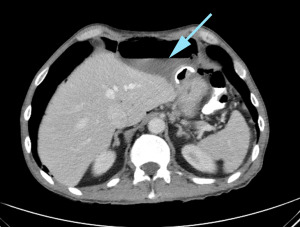
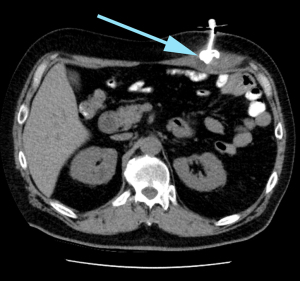
Over the 90-day post-placement period there was a total of 3 (3%) major complications. These included solid organ injury (n=1, liver) (Figure 1), intra-abdominal abscess (n=1) (Figure 2) and buried bumper syndrome (n=1) (Figure 3). There was a total of 6 (6%) minor complications associated with the procedure; transient paralytic ileus (n=2), peristomal leakage (n=3), and intramuscular haematoma (n=1). There were no procedure related mortalities. Patient and tumour factors were similar between those who had a complication and those whose PEG insertion was uneventful (Table 1). Time to PEG insertion was significantly shorter in the group with complications compared to uneventful PEG placement (2.3±2.7 vs. 1.2±0.9 days P=0.01). The number of concurrent procedures were similar between those patients with complications and uneventful PEG insertion (60% vs. 67%, P=0.74) (Table 1).
Discussion
Gastroenterologist and general surgeons continue to provide an excellent service for insertion of PEG tubes in all populations. Head and neck cancer patients are deemed to be a unique patient cohort that significantly differs to the standard population of PEG tube recipients. Their tumour or post treatment related changes add anatomical distortion and complexity to the view and instrumentation when accessing the upper oesophagus. It is in this region the OLR-HN surgeon is most comfortable. Indeed, the OLN-HN surgeons are integral in the assessment, diagnosis, and management of these patients within the multidisciplinary team. As Ruohoalho has suggested, the potential for placement of a PEG tube whilst simultaneously undertaking other similarly anatomically located procedures is beneficial. In our instance 39% of cases undertook a concurrent procedure, predominantly involving either the assessment, diagnosis, or definitive surgical management of their cancer. For those without a concurrent procedure, the continuity of care of the otolaryngology surgical team and low wait time (2.2±2.6 days) supports the potential holistic and logistical benefits that the OLN-HN driven PEG service may provide.
Success rate of insertion of PEG tubes by an OLN-HN surgeon in this study (100%), had similar outcomes compared to the previously described literature for PEG tubes inserted by gastroenterologists and general surgeons (97.6–98.9%) (2,6).
Major (3%), minor (6%) and overall (9%) complication rates demonstrated in this paper is comparable both in nature and numbers to previously described studies of similar populations (major complication rates 2–13.9%, minor rates 11–35%) (2,3,6,8,9). Major complications in our study included a solid organ injury (subcapsular haematoma of the liver), in a patient who commenced on dual antiplatelet therapy following a cardiac stent placement 3 days post PEG placement. A further patient had an intraabdominal abscess that required hospital admission, intravenous antibiotics, and laparoscopic drainage of an abscess between the stomach and anterior abdominal wall. This patient had a significant cricopharyngeal stenosis requiring a dilatation to pass a paediatric gastroscope. Due to this difficulty, a repeat gastroscopy to confirm position of the bumper was not performed at the time of insertion. Finally, one patient experienced buried bumper syndrome; a condition where the bumper of the PEG tube migrates into the gastric wall. In this case, the PEG was removed, and a new PEG was placed within 48 hours. Major complications of this nature have been well documented for as long as PEG insertion has been performed (7).
Minor complications included three patients with peristomal leakage, all were low volume and self-resolving following stoma care. No tubes required replacing. Two patients suffered from a temporary paralytic ileus which resolved with conservative care, including temporary cessation of feeds. Our final complication was in a patient who developed an intramuscular haematoma of the rectus sheath, requiring readmission and intravenous antibiotics. Whilst our minor morbidity rate is low compared to the reported literature, studies differ in their definition of complications and the subjectivity of assessment. Other factors that could contribute to our rate include having regular surveillance and care by an experienced stoma therapist and head and neck trained dietician. However, the retrospective nature of the study with reliance on accurate contemporaneous documentation may lead to an underestimation of minor side effects.
Overall, there was no significant difference between patient, tumour and insertions factors with regards to complication rate. However, the significant increase in complication rate with regards to shorter time to insertion may reflect the overall severe health status and possibly reflect a worse prognosis overall for these patients. Given the small numbers in the complication groups, comparisons between the complication and uneventful groups may be underpowered and thus meaningful factors may be overlooked. This is a limitation of this study which may be overcome with a larger sample size.
Conclusions
Otolaryngologists play an integral part in the assessment, diagnosis and management of head and neck cancer patients. The effectiveness of an otolaryngology driven PEG placement has been described and the associated morbidity in an Australian head and neck cancer centre reviewed. Success and complication rates were comparable to those previously reported in head and neck cancer patients. Additional benefits of this style of PEG service may include logistical benefits including simultaneous insertion of PEG tubes with concurrent procedures and improved continuity of care.
Acknowledgments
The authors would like to thank the reviewers for their critical analysis and valuable comments which improved the quality of this paper. We would also like to thank Hao-Wen Sim (The Kinghorn Cancer Centre), and Raquel Alvarado (ENT Clinic Sydney) for their advice and guidance in data and statistical analysis.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/ajo-21-33
Data Sharing Statement: Available at https://dx.doi.org/10.21037/ajo-21-33
Peer Review File: Available at https://dx.doi.org/10.21037/ajo-21-33
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/ajo-21-33). JAC serves as an unpaid editorial board member of Australian Journal of Otolaryngology from January 2021 to December 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Adequate protection of patient privacy and confidentiality was ensured through deidentification and appropriate data storage. Consent was justifiably waived. Research ethics board approval was granted for the study protocol by St. Vincent’s Hospital Human Research Ethics Committee (PID01240).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Selz PA, Santos PM. Percutaneous endoscopic gastrostomy. A useful tool for the otolaryngologist-head and neck surgeon. Arch Otolaryngol Head Neck Surg 1995;121:1249-52. [Crossref] [PubMed]
- Ruohoalho J, Aro K, Mäkitie AA, et al. Prospective experience of percutaneous endoscopic gastrostomy tubes placed by otorhinolaryngologist-head and neck surgeons: safe and efficacious. Eur Arch Otorhinolaryngol 2017;274:3971-6. [Crossref] [PubMed]
- Bäck LJ, Benders A, Pietarinen P, et al. Percutaneous endoscopic gastrostomy tube placement by otorhinolaryngologist-head and neck surgeons. Acta Otolaryngol 2014;134:760-7. [Crossref] [PubMed]
- Amin MB, Edge SB, Greene FL, et al. editors. AJCC Cancer Staging Manual. 8th ed. Chicago: American College of Surgeons, 2017.
- Ponsky JL, Gauderer MW. Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy. Gastrointest Endosc 1981;27:9-11. [Crossref] [PubMed]
- Bannister M. Insertion of percutaneous endoscopic gastrostomy by head and neck surgeons: systematic review. Br J Oral Maxillofac Surg 2016;54:132-4. [Crossref] [PubMed]
- Schapiro GD, Edmundowicz SA. Complications of percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am 1996;6:409-22. [Crossref] [PubMed]
- Grant DG, Bradley PT, Pothier DD, et al. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol 2009;34:103-12. [Crossref] [PubMed]
- Kao SS, Marshall-Webb M, Dharmawardana N, et al. Gastrostomy tube insertion outcomes in South Australian head and neck cancer patients. Aust J Otolaryngol 2018;1:21. [Crossref]
Cite this article as: Morris CL, Kornfeld B, Singh R, Leavers BC, Gallagher RM, Crawford JA. Otolaryngology driven percutaneous endoscopic placement of gastrostomy tubes as part of integrative head and neck cancer service. Aust J Otolaryngol 2021;4:36.


