
Management of petrous temporal bone fractures: a 5-year experience from an Australian major trauma centre
Introduction
Fractures of the petrous temporal bone (PTB) usually results from high energy head trauma and hence are often associated with substantial intracranial injuries that remain the priority in the initial treatment period (1). As more patients are surviving from severe traumatic brain injury (TBI), it would be expected more patients will be living with the sequalae from their PTB fracture.
Complications after PTB fracture reflect the complex anatomy of this bone and the important structures encased within it, and can include traumatic facial nerve palsy (FNP), cerebrospinal fluid (CSF) leak and varying degrees of hearing loss. There has been ongoing debate regarding the classification of these fractures, however they are increasingly being classified according to their involvement of the otic capsule (bony labyrinth) as this has been shown to be more prognostic (2).
This study aims to describe the incidence of PTB fractures in an Australian adult population and their complications presenting to a level-one major trauma centre in Melbourne, Australia. In addition, we develop an evidence-based clinical practice guideline for their management. We present the following article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-22-7/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. After local ethical review board approval (QA2012012) we conducted a retrospective case series of all patients with a PTB fracture from a single level-one Tertiary Major Trauma Service in Melbourne, Victoria. Patients were identified using the Royal Melbourne Hospital Trauma Registry (RMH-TR) over the 5-year period between October 2015–October 2020. Patients were excluded if they were under 16 years old, had incomplete medical records or temporal bone fractures not involving the petrous portion.
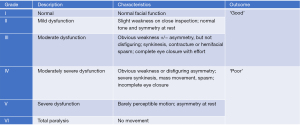
Data was extracted from electronic medical records and RMH-TR for demographics, mechanism of injury, length of hospital stay, mortality, radiologic characteristics, Injury Severity Score (ISS), and associated injuries. Additional data was collected on PTB fracture-related complications including blunt cerebrovascular injury (BCVI), traumatic FNP and CSF leak was also collected. Subsequent hearing outcomes were assessed at first follow-up (average 12.6 weeks) using audiology data where available. Fractures were classified using the traditional ‘longitudinal vs. transverse vs. mixed’ system as well as the newer ‘otic capsule violating (OCV) vs. otic capsule sparing (OCS)’ classification. Facial nerve (CN7) outcomes were graded using the House-Brackmann equivalent (HB) scale with grade 1–3 classified as ‘good’ (signifying non-disfiguring weakness and complete eye closure), and grade 4–6 classified ‘poor’ (Figure 1). Patients with FNP had their images reviewed by a lateral skull base otologist (CI) to determine if there was a visible fracture line that appeared to transgress the pathway of CN7, and if so which section(s) were involved.
Statistical analysis
Data was recorded using REDcap database. Statistical analysis was performed using IBM SPSS v20 for both descriptive and comparative testing. Ordinal variables were compared using chi-squared or Fishers exact analysis. Multivariable logistic regression was performed to look for predictors of OCV fractures. Significance level for any given P value was set at 0.05.
Results
Patient demographics
Over the study period 22,479 patients were referred to the RMH trauma service. Of these, 377 patients had a total of 419 PTB fractures (42 bilateral injuries). Most patients were male (78.2%) with a median age of 42 years (IQR, 27–64 years). The most common mechanism of injury was a fall (52.5%) with 32.1% resulting from a fall from standing height (<1 m) (median age 63 years; IQR, 42–79) and 20.4% from a high fall (>1 m) (median age 56 years; IQR, 31–74). The next most common mechanism was road traffic crashes including pedestrian vs. car (12.2%), motor vehicle crashes (MVC) 10.3%, motorbike crashes (MBC) 8.5%, and cyclist injuries 4%. The remainder were blunt head injuries of various nature, including 9.3% assaults (Table 1).
Table 1
| Characteristic | Patients (N=377), n (%) |
|---|---|
| Age (years), median [IQR] | 42 [27–64] |
| Sex | |
| Male | 295 (78.2) |
| Female | 82 (21.8) |
| Mechanism of injury | |
| All falls | 198 (52.5) |
| Fall <1 m | 121 (32.1) |
| Fall >1 m | 77 (20.4) |
| Ped vs. car | 46 (12.2) |
| MVC | 39 (10.3) |
| Assault | 35 (9.3) |
| MBC | 32 (8.5) |
| Cyclist | 15 (4.0) |
| Other | 7 (1.9) |
| Sport | 4 (1.1) |
| Penetrating | 1 (0.3) |
| Hospital length of stay (days), median [IQR] | 7 [3–16] |
| Intubated within first 24 h | 171 (45.4) |
| Admitted ICU | 181 (48.0) |
| Ventilation, median [IQR] | 3 [1–8] |
| Major trauma (ISS >12) | 301 (79.8) |
| Severe TBI (GCS <9) | 121 (32.1) |
| Imaging | |
| CT carotid angiogram | 245 (58.5) |
| CT-TB (reformats) | 80 (19.1) |
| CT-TB (dedicated) | 17 (4.1) |
| Death | 64 (17.0) |
| Disposition | |
| Home | 148 (47.3) |
| Rehabilitation centre | 147 (47.0) |
| Other | 18 (5.7) |
IQR, interquartile range; MVC, motor vehicle crashes; MBC, motor bike crashes; ICU, Intensive Care Unit; ISS, Injury Severity Score; GCS, Glasgow Coma Score; TBI, traumatic brain injury; CT-TB, CT temporal bone.
Of the patients admitted with a PTB fracture, 79.8% were classified as major trauma (ISS >12). One third (32.1%) suffered a severe TBI. On initial assessment, 45.5% required intubation, precluding proper assessment of their CN7 function, with 48% admitted to the Intensive Care Unit (ICU). Median time ventilated was 3 days (IQR, 1–8 days). The median length of stay (LOS) in hospital was 7 days (IQR, 3–16 days). Sixty-four patients (17%) died during their admission, all due to unsurvivable head injury. Of those who survived (n=313), 47.3% were discharged home, with the remainder transferred to rehabilitation centre (47%) or other hospital facilities (5.7%) (Table 1).
PTB injury characteristics
There were 243 patients (64.5%) with additional base of skull (BOS) fracture/s and 84.9% had associated intracerebral haemorrhage (ICH). There were 71.4% longitudinal fractures, 22.9% transverse and 5.7% mixed or comminuted fractures. Out of the 419 fractures, 5.3% were OCV with the remaining 94.7% being OCS. The carotid canal was involved in 30.5% of fractures and 7.6% involved the facial nerve canal. Ossicular chain discontinuity (OCD) was seen in 6.9% of fractures (Table 2).
Table 2
| Characteristic | n (%) |
|---|---|
| Total fractures | 419 |
| Side | |
| Right | 201 (48.0) |
| Left | 218 (52.0) |
| Bilateral | 42 (11.1) |
| Other BOS fracture | 243 (64.5) |
| ICH | 320 (84.9) |
| Classification 1 | |
| Longitudinal | 299 (71.4) |
| Transverse | 96 (22.9) |
| Mixed | 24 (5.7) |
| Classification 2 | |
| OCV | 22 (5.3) |
| OCS | 397 (94.7) |
| Involving carotid canal | 128 (30.5) |
| Involving facial nerve canal | 32 (7.6) |
| OCD | 29 (6.9) |
BOS, base of skill; ICH, intracerebral haemorrhage; OCV, otic capsule violating; OCS, otic capsule sparing; OCD, ossicular chain discontinuity.
Complications
BCVI
There were 22 cases (9%) of BCVI out of the total of 245 injuries (58.5%) that underwent CT carotid angiography. Most were Denver grade 1 injuries (54.5%) (3). Ten (22.7%) of these patients were managed with antiplatelet agents and 2 (9%) with anticoagulants. When these injuries were present, there was a mortality rate of 45%, with 22.7% developing neurological sequalae (Table 3). Involvement of the carotid canal was significantly increased in OCV fractures (82% vs. 28%; OR 10.4; P≤0.001), but not with the rate of BCVI.
Table 3
| Characteristic | n (%) |
|---|---|
| CN7 injury | 39 (9.3) (37 patients) |
| Bilateral FNP | 2 (5.4) |
| Onset | |
| Immediate | 9 (23.1) |
| Delayed | 30 (76.9) |
| Intubated preventing assessment | 20 (51.3) |
| CN7 section involved | |
| Meatal | 3 (9.4) |
| Labyrinth | 4 (12.5) |
| Geniculate | 11 (34.4) |
| Tympanic | 22 (68.8) |
| Mastoid | 9 (28.1) |
| Nerve conduction tests (ENOG/EMG) | 7 (17.9) |
| Management | |
| None | 13 (33.3) |
| Steroids (any) | 26 (66.7) |
| Mean duration steroid | 7 days |
| Eye complications | 9 (23.1) |
| Outcome (n=32) | |
| Good (HB 1–3) | 26 (81.3) |
| Bad (HB 4–6) | 6 (18.8) |
| Total CSF leak | 53 (12.6) |
| Otorrhoea | 44 (83.0) |
| Rhinorrhoea | 15 (28.3) |
| Average duration of leak | 7 days |
| Management | |
| Lumbar puncture | 2 (3.8) |
| Lumbar drain | 5 (9.4) |
| Average timing of drain | Day 7 |
| Antibiotic cover for CSF leak | 26 (49.1) |
| Meningitis | 2 (3.8) |
| Hearing loss type (n=115) | |
| None | 27 (23.5) |
| CHL | 20 (17.4) |
| SNHL | 50 (43.5) |
| Mixed HL | 18 (15.7) |
| Extent hearing loss (n=115) | |
| None | 27 (23.5) |
| Mild | 33 (28.7) |
| Moderate | 22 (19.1) |
| Mod-severe | 6 (5.2) |
| Severe | 9 (7.8) |
| Profound | 18 (15.6) |
| Hearing management | |
| Referred for hearing aids | 23 (7.3) |
| Referred for cochlear implant/other | 3 (1.0) |
| Surgery | 3 (1.0) |
| Carotid injuries | 22 (9.0) |
| Denver grade | |
| Grade 1 | 12 (54.5) |
| Grade 2 | 4 (18.2) |
| Grade 3 | 3 (13.6) |
| Grade 4 | 3 (13.6) |
| Management | |
| Conservative | 5 (22.7) |
| Antiplatelet | 10 (45.5) |
| Anticoagulant | 2 (9.0) |
| Endovascular | 0 |
| Outcome | |
| Neurological sequalae | 5 (22.7) |
| Death | 10 (45.5) |
PTB, petrous temporal bone; CN7, facial nerve; FNP, facial nerve palsy; ENOG, electroneuronography; EMG, electromyography; HB, House-Brackmann; CSF, cerebrospinal fluid; CHL, conductive hearing loss; SNHL, sensorineural hearing loss.
Facial nerve injury
There were 39 cases of traumatic FNP (9.3%). Nine were clearly immediate onset and 30 delayed onset. Almost half of the patients in the delayed category were intubated pre-hospital or in the emergency department precluding assessment of facial nerve function in the first 24 h. There were 2 patients (5.4%) with bilateral injuries. Nine (23%) injuries resulted in eye complications. OCV fractures were associated with an increased risk of FNP (27% vs. 8%; OR 3.7; P=0.01). Nerve conduction studies (ENOG/EMG) were performed in 7 cases (18%). No patients underwent surgery on their facial nerve in our cohort. Twenty-six patients with FNP (66.7%) were treated with steroid therapy. Patients received either oral prednisolone or intravenous dexamethasone for an average duration of 7 days. The remaining 13 injures (33.3%) had no treatment.
Of the injuries with documented follow up (n=32), 26 injuries (81.3%) achieved a good HB outcome (HB 1–3), while the remaining 6 injures (18.8%) had a poor outcome (HB 4–6). A total of 12 injuries (37.5%) achieved complete return of normal function (HB 1). The median follow-up time from injury was 11.6 weeks (IQR, 7.6–21.2 weeks). We found no significant difference in outcome between patients who were treated with steroids compared to those who did not (P=0.37). When considering which part of the nerve was crossed by the fracture line, the tympanic segment was the most affected (68.8%) followed by the geniculate area (34.4%) (Table 3).
CSF leak
There were a total of 53 CSF leaks (12.6%) with the majority presenting with otorrhoea. The average duration of leak was 7 days with 2 patients reporting further intermittent rhinorrhoea after discharge. Five patients (9.4%) required a lumbar drain with resolution of the leak on average after 7 days. Antibiotics were administered for 26 patients (49%). Two patients developed meningitis (both of whom had received antibiotics).
Hearing loss
Of the 313 patients who survived their admission, audiology testing was conducted for one-third (n=115; 37%). Sensorineural hearing loss (SNHL) was seen in 50 injuries (43%), conductive hearing loss (CHL) in 17% and 16% with a mixed picture. Only 23% had no hearing loss. There was a statistically significant association between having an OCV fracture and SNHL pattern (88% vs. 40%; OR 10.1; P≤0.05) which was more likely to be profound in nature (88% vs. 10%; OR 66.9; P≤0.001) (Table 4). Having an ossicular chain injury was not significantly associated with a CHL. Of the 55 patients with at least moderate hearing loss, twenty-three (42%) were referred for hearing aids, three (5%) referred for cochlear implants and three (5%) for ossicular reconstructive surgery.
Table 4
| Variable | Univariable (unadjusted) | Multivariable logistic regression analysis adjusted for age & sex | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n=419) | OCV (n=22) | OCS (n=397) | P value | OR | 95% CI lower | 95% CI higher | P value | ||
| Male | 332 [79] | 21 [96] | 311 [78] | 0.088 | 1.76 | 0.77 | 43.79 | 0.088 | |
| Age (years) (median) | 40 | 39 | 40 | 0.202 | |||||
| Severe TBI | 159 [38] | 16 [73] | 143 [36] | 0.002 | 4.40 | 1.67 | 11.61 | 0.003 | |
| Death | 76 [18] | 9 [41] | 67 [17] | 0.007 | 4.62 | 1.80 | 11.84 | 0.001 | |
| ICH | 356 [85] | 22 [100] | 334 [84] | 0.997 | |||||
| BOS fracture (any) | 285 [68] | 20 [91] | 265 [67] | 0.032 | 4.41 | 1.01 | 19.27 | 0.049 | |
| Frontal | 55 [13] | 7 [32] | 48 [12] | 0.011 | 3.16 | 1.21 | 8.25 | 0.019 | |
| Sphenoid | 172 [41] | 10 [45] | 162 [41] | 0.667 | |||||
| Occipital | 145 [35] | 14 [64] | 131 [33] | 0.005 | 3.50 | 1.42 | 8.60 | 0.006 | |
| Contralateral PTB | 103 [25] | 6 [27] | 97 [24] | 0.764 | |||||
| Fracture class 1 | |||||||||
| Longitudinal | 299 [71] | 5 [23] | 294 [74] | <0.001 | 0.11 | 0.04 | 0.31 | <0.001 | |
| Transverse | 96 [23] | 12 [55] | 84 [21] | <0.001 | 4.20 | 1.74 | 10.13 | 0.001 | |
| Mixed | 24 [6] | 5 [23] | 19 [5] | 0.002 | 5.23 | 1.71 | 15.94 | 0.004 | |
| BCVI | 22 [11] | 2 [9] | 20 [5] | 0.452 | |||||
| Traumatic FNP | 39 [9] | 6 [27] | 33 [8] | 0.006 | 3.77 | 1.35 | 10.51 | 0.011 | |
| CSF leak | 53 [13] | 5 [23] | 48 [12] | 0.153 | |||||
| OCD | 29 [7] | 5 [23] | 24 [6] | 0.043 | 3.22 | 0.10 | 10.41 | 0.051 | |
| Involving CN7 canal | 32 [8] | 12 [55] | 19 [5] | <0.001 | 21.91 | 8.25 | 58.22 | <0.001 | |
| Involving carotid canal | 128 [31] | 18 [82] | 110 [28] | <0.001 | 10.43 | 3.43 | 31.74 | <0.001 | |
| Hearing loss type (n=115) | 115 | 8 | 107 | ||||||
| None | 27 [23] | 1 [13] | 26 [24] | 0.459 | |||||
| CHL | 20 [17] | 0 [0] | 20 [19] | 0.998 | |||||
| SNHL | 50 [43] | 7 [88] | 43 [40] | 0.031 | 10.10 | 1.17 | 86.92 | 0.035 | |
| Mixed HL | 18 [16] | 0 [0] | 18 [17] | 0.998 | |||||
| Extent Hearing loss (n=115) | 115 | 8 | 107 | ||||||
| None | 27 [23] | 1 [13] | 26 [24] | 0.459 | |||||
| Mild | 33 [29] | 0 [0] | 33 [31] | 0.998 | |||||
| Moderate | 22 [19] | 0 [0] | 22 [21] | 0.998 | |||||
| Mod-severe | 6 [5] | 0 [0] | 6 [6] | 0.999 | |||||
| Severe | 9 [8] | 0 [0] | 9 [8] | 0.999 | |||||
| Profound | 18 [16] | 7 [88] | 11 [10] | <0.001 | 66.86 | 6.75 | 662.49 | <0.001 | |
| Assoc symptoms (n=313) | 313 | 12 | 301 | ||||||
| Tinnitus | 38 [12] | 2 [17] | 36 [12] | 0.626 | |||||
| Vertigo | 38 [12] | 5 [42] | 33 [11] | <0.001 | 5.61 | 1.65 | 19.01 | 0.006 | |
The data are expressed as n [%]. OCV, otic capsule violating; OCS, otic capsule sparing; TBI, traumatic brain injury; ICH, intracerebral haemorrhage; BOS, base of skull; BCVI, blunt cerebrovascular injury; FNP, facial nerve palsy; CSF, cerebrospinal fluid; OCD, ossicular chain discontinuity; CN7, facial nerve; CHL, conductive hearing loss; SNHL, sensorineural hearing loss; OR, odds ratio; CI, confidence interval.
Other complications
A significant number of patients with OCV fractures suffered vertigo (12%) in the weeks after their injury (P<0.05) and although similar number suffering from tinnitus this was not significant between the fracture groups. There were infrequently reported other complications including loss of taste (3.5%), tympanic membrane (TM) perforation (1.9%) and ear canal stenosis (0.6%) (Table 3).
Discussion
This study of PTB fractures from an adult major trauma service is the first to describe these injuries in an Australian adult population and is one of the largest series to date. Previous studies have shown that PTB fractures require high energy transfer (up to 8,000 newtons of force from cadaveric studies) and have historically been associated with MVC’s (1). However, in this series, we found falls to be the most common mechanism (52.5%) which appears to be an emerging trend in the literature (4,5). Interestingly, we found that 20.4% occurred after low-falls (from standing height) which has not previously been reported. The reason for this is unclear but could in part be attributed to the ageing population as the median age for these patients was 20 years greater than the median age for the entire population (42 vs. 63 years).
PTB fractures have traditionally been classified anatomically based on the orientation of the fracture line with respect to the long axis of the petrous bone (longitudinal, transverse or mixed). Longitudinal fractures are more common (50–80%) (6), with similar rate seen in this study of 71.4%. Although intuitive, this classification has previously been scrutinised for its relevance as it has shown no correlation with clinical outcomes and does not provide useful prognostic information (2,7). We found that transverse fractures were more likely to be OCV (P≤0.001), with longitudinal fractures more likely to be OCS (P≤0.001), but we found no significant association with the traditional anatomical classification and complications (Table 4). This study adds to the growing body of evidence that would suggest this classification system be abandoned in future research and clinical practice.
Classification of PTB fractures defined by involvement of the otic capsule, has previously shown to give more clinically relevant prognostic information. OCV fractures invariably result in some degree of SNHL and are associated with increased risk of other complications, including traumatic FNP, CSF leak and intracranial injury (2,8-10). While OCS fractures are more likely to result in CHL (2,7,8).
In our study we found 5.3% were OCV which is in keeping with the literature (2–7%) (6). We found OCV fractures were more likely to result in increased rates of CN7 injury (P≤0.001), SNHL (P≤0.05) which is more likely to be profound (P≤0.001) and vertigo (P≤0.05). We also found OVC fractures were more likely to be associated with other BOS fractures (frontal & occipital), severe TBI, and death. However, these are likely independent and attributable to the significant forces required to cause the injury. Our findings support the continued move to classify PTB fractures based on the involvement of the otic capsule.
Facial nerve injury
Traumatic FNP was seen in 9.3% of our cohort, with a range of around 7–15% reported in the literature (9-12). OCV fractures are associated with up to 5 times higher risk of FNP compared to OCS fractures (2). This was similarly seen in our population (27% vs. 8%; OR 3.2; 95% CI: 9.5–69.1; P≤0.001). It is typically classified into immediate onset FNP happening at the time of injury and being obvious on initial presentation vs. delayed onset with paralysis occurring over subsequent days. It can be difficult to make this important distinction when patients are intubated and these patients should be considered and managed as an immediate injury (1,13). FNP can be complete or incomplete, with the latter suggesting a neuropraxia and generally having an excellent rate of complete recovery with conservative treatment (9,14,15).
The onset of paralysis is important because as it can indicate the severity of the nerve damage. Immediate onset FNP is due to compression of the nerve from bony fragments or in worst case scenario complete nerve transection. Delayed onset palsy is thought to be due to contusion or perineural haematoma causing neural oedema and compression against the bony canal (6). Previous studies have also found generally good recovery for delayed injuries with conservative management (9,15-18). This usually consists of a 1–2-week course of high dose steroid therapy, if not contraindicated (1). This evidence comes mostly from the management of idiopathic FNP and there is limited evidence for its use in traumatic facial nerve injury, but it is still standard practice (19). All patient with FNP were managed non-surgically with or without steroids, and despite small sample size we found no statistically significant difference in those achieving a good outcome when steroids were administered (P=0.37). Steroids must be used with caution in the severely head injured patient after the result of the CRASH study and Cochrane review finding increased mortality in this cohort (20,21).
We found in total 6 injuries (18.8%) had a poor outcome (HB 4–5) in our study. Only two of these cases had nerve conduction performed and adequate follow-up to draw conclusions. It is unclear whether these cases were offered surgery, but their outcomes suggest a severe facial nerve injury that may have benefited from exploration. Surgical intervention for patients with facial nerve injury remains controversial, as does the timing of surgery. Some authors recommend early decompression within 2–4 weeks after injury (13,22). However, recent studies have suggested there is still benefit from delayed surgery 1–3 months after injury (5,15,23,24). Several authors agree there are considerably worse outcomes after 3 months (15,22).
Patients with immediate or complete FNP are generally considered to have a worse nerve injury consistent with axonotmesis or neurotmesis and it is recommended these patients are assessed with nerve testing using either electroneuronography (ENoG) or electromyography (EMG). The primary benefit of EnoG is for prognostication and it has been generally accepted that EnoG showing >90% degeneration of the affected side in the first 6 days or progressing to >95% within 14 days is associated with a poor recovery and should be an indication for surgery (9,23,25). Other studies have shown that ENOG <90% is associated with a good outcome and can be managed conservatively (14). The decision to perform nerve conduction studies will vary widely between hospitals and countries, depending on availability of testing and surgeon preference. Patients may also be referred late to specialist centres, having missed the relatively short window where these tests are most useful. However, with the global trend for trauma patients to be managed in specialist tertiary centres with multi-disciplinary team input, these tests should be more accessible for early assessment.
CSF leak
It has been reported that up to 78% of traumatic CSF leaks will resolve spontaneously within 7 days with conservative management (9), which involves a period of bed rest and measures to prevent fluctuations in intracranial pressure and blood pressure (such as avoiding nose blowing, straining etc.) (26). This is usually appropriate until day 7–10 at which point further intervention may be required with lumbar puncture (LP) or lumbar drain (1). One study reported a significant reduction in the duration of CSF leak of 3.4 days for patients treated with lumbar drain compared to conservative management alone (27). The average duration of leak in our study was 7 days with 2 patients undergoing LP and 5 patients requiring a lumbar drain. None required specific surgical intervention during the study period emphasising the majority will heal.
One of the well documented risks after CSF leak is the possibility of meningitis. Meningitis is rare in patients without CSF leak and antibiotics are not indicated as prophylaxis for BOS fracture alone (28). In our study 49.1% of patients with a CSF leak were on antibiotics, but only 2 patients (3.8%) with a leak developed meningitis, both having received antibiotic therapy. Previous meta-analyses found conflicting results for the risks of meningitis after traumatic CSF leak (29,30). To address these deficiencies, two Cochrane database meta-analysis performed in 2011 and 2015 which included five randomized controlled trials have since concluded that the evidence does not support the use of prophylactic antibiotics to reduce the risk of meningitis in these patients (28,31). This is increasingly important in the modern era of global antibiotic resistance, and the data from our study tentatively supports this conclusion although patients may require antibiotics for other injuries. Previous studies have shown the rate of meningitis is increased in prolonged CSF leaks over 7 days so patients should receive active management if persistent beyond this point (9).
Hearing loss
Hearing loss is reported to affect between 24–81% of patients after PTB fracture (8,9). Most patients will complain of some degree of hearing loss which may be transient or permanent. Injury can result in a SNHL, CHL or mixed picture. SNHL implies there has been damage to the delicate structures of the inner ear whereas CHL implies an issue lateral to the cochlea, such as haemotympanum, TM perforation or damage to the ossicular chain (6).
CHL second to haemotympanum is expected to resolve in the weeks after injury and TM perforations should be monitored for resolution. A 30-dB air-bone gap that persists after 6–8 weeks would be suspicious for an ossicular injury and should be considered for middle ear exploration (1). Conservative management of CHL often leads to spontaneous recovery and intervention is rarely needed before 6 months (32). Damage to ossicles is seen more often in longitudinal fractures, with directional classification schemes less predictive of SNHL (6).
There is a stronger association between OCV fractures and profound SNHL from the literature, with OCV fractures up to 25 times more likely to cause SNHL (2,8,9). This is supported by our study whereby OCV fractures were 2 times more likely to result in SNHL (88% vs. 40%; P≤0.05) and 4 times more likely to be profound in nature (88% vs. 10%; P<0.001). We found no statistically significant association between hearing outcomes and directional classification in our population.
SNHL can be immediately apparent but is usually difficult to assess unless the patient is neurologically stable. If possible, early detection is beneficial and initiation of steroid therapy may help preserve hearing function as shown by Padmakumar et al. where early steroids caused a mean improvement in hearing of 12.7 dB (33). However, hearing loss is often not assessable soon after injury and the same cautions with steroids in severe TBI mentioned previously apply.
Other symptoms
There are a number of other sequalae from PTB fracture that were seen in our study that have also been well documented in other series (9,10). Of the cases surviving for follow up (n=313), there were 38 with vertigo (12%), 38 with tinnitus (12%), 11 with anosmia (3.5%) and 13 patients with loss of taste (4.2%). Whilst not the focus of this study, these are symptoms that can negatively impact a patient’s quality of life and should be monitored for in the follow up period. Fracture of the tympanic segment and damage to the bony ear canal can lead to canal stenosis, which occurred in 2 patients in this series. Early use of ear wicks or packing can splint the canal to help prevent this complication, as it can be difficult to treat once established and can predispose to later cholesteatoma (1).
Imaging
The recommended imaging for accurate detection of PTB fracture is multidetector CT temporal bone (CT-TB) with multiplanar reconstructions at a section thickness of 1mm or less. It is generally recommended that patients with complex radiological fractures or clinical complications from their PTB fracture should have dedicated fine slice imaging to assess their need for intervention (34). Not all PTB fractures may be seen on initial imaging studies and in trauma patients with occult signs of fracture, these can again be further assessed with dedicated CT-TB imaging. However, several studies have suggested that dedicated imaging may not be required for all patients. The combined use of head, maxillofacial and cervical spine CT, which is a common imaging series in head trauma, has been shown to be sufficient to diagnose 99.0% of PTB fractures compared to dedicated CT-TB (4). The negative predictive value (NPV) of maxillofacial CT alone vs. CT-TB to diagnose carotid canal involvement was reported as >95% (35).
Temporal bone reformats were requested in 80 patients (19.1%) in this study, with only 17 patients (4.1%) having a dedicated fine slice CT temporal bone. A recent analysis by Szczupak et al. showed that in low-risk patients (without involvement of major temporal bone structures or clinically apparent complications) the standard head trauma CT protocol (as performed at our centre) had a NPV of 100% for OCV or CN7 injury compared to dedicated CT-TB, and NPV of 96% and 99% for carotid canal involvement and OCD respectively (36). They suggested that in this patient group standard head trauma CT protocols are sufficient to diagnose PTB fractures and exclude major complications. This also limits exposure to unnecessary ionising radiation, reduces radiology burden and in-hospital transport of critically ill patients and provides a significant cost saving to the health service (36). We would advocate that reformatted PTB images from the initial head trauma CT series are sufficient for most patents unless they have clinical complications from their temporal bone injury that should then be further assessed with dedicated CT-TB or if complications become apparent on follow-up.
This low rate of dedicated imaging is due to all trauma patients at our centre receiving 1mm slices through the BOS during initial trauma imaging, and in part related to logistic difficulties of transporting patients from ICU and radiology access in a busy tertiary trauma centre. With low patient numbers we were unable to make comparisons between the imaging modalities.
Limitations
This study had some limitations primarily in its retrospective design and accessing information from medical records that at times can be incomplete. Patient selection from the trauma registry at a major tertiary referral centre, which is the second busiest in Australia, means almost all patients had other critical injuries that may factor to overestimate mortality rates. Only patients meeting the trauma registry criteria were recorded in the registry with a complete dataset allowing them to be included in this study, again possibly overestimating the incidence of PTB fracture amongst the general head injury population. Conversely, due to the relatively high in-patient mortality rate and incomplete audiology follow up, not all patients could be properly assessed for complications, so these may be underestimated in our study. Modern understanding of TBI suggests an evolving model of injury including duration of post traumatic amnesia to diagnose severity. We used the original definition of severe TBI (GCS <9) and this may under- or over-estimate this group.
Clinical practice guideline
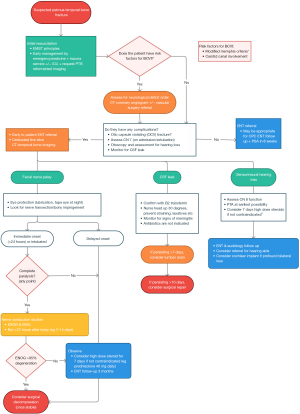
Using the findings published in this study and published international literature we developed an evidence-based clinical practice guideline for the management of temporal bone fractures at our centre that we think is suitable for other centres that may not have their own guideline (Figure 2).
Conclusions
PTB fractures usually occur after significant head trauma, however, should still be suspected in ‘lower’ mechanism injuries such as a fall from standing height, particularly in the elderly. They often occur in poly-trauma patients and are associated with severe TBI and the initial management strategies should focus on stabilisation of these other injuries. Early ENT input should be sought in cases of FNP and CSF leak to facilitate specialised investigation and intervention. Most other injuries including hearing loss can be managed on an outpatient basis. Standard head trauma CT imaging with reformatted PTB images should be adequate in most situations unless there is an OCV fracture or PTB specific complications, in which case dedicated CT-TB imaging should be sought. We advocate that only the otic capsule classification system be used in future clinical practice and research as it better predicts clinical outcomes. We introduce an evidence-based clinical practice guideline for their management at our centre, which could be used and any similar-level trauma centres around the world.
Acknowledgments
I would like to acknowledge the administrators of the RMH trauma registry for their help collecting this data.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-22-7/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-22-7/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-22-7/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-22-7/coif). CI serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflict of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by local institutional ethics committee (QA2012012) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Diaz RC, Cervenka B, Brodie HA. Treatment of Temporal Bone Fractures. J Neurol Surg B Skull Base 2016;77:419-29. [Crossref] [PubMed]
- Little SC, Kesser BW. Radiographic classification of temporal bone fractures: clinical predictability using a new system. Arch Otolaryngol Head Neck Surg 2006;132:1300-4. [Crossref] [PubMed]
- Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma 1999;47:845-53. [Crossref] [PubMed]
- Schubl SD, Klein TR, Robitsek RJ, et al. Temporal bone fracture: Evaluation in the era of modern computed tomography. Injury 2016;47:1893-7. [Crossref] [PubMed]
- Ulug T, Arif Ulubil S. Management of facial paralysis in temporal bone fractures: a prospective study analyzing 11 operated fractures. Am J Otolaryngol 2005;26:230-8. [Crossref] [PubMed]
- Kennedy TA, Avey GD, Gentry LR. Imaging of temporal bone trauma. Neuroimaging Clin N Am 2014;24:467-86. viii. [Crossref] [PubMed]
- Ishman SL, Friedland DR. Temporal bone fractures: traditional classification and clinical relevance. Laryngoscope 2004;114:1734-41. [Crossref] [PubMed]
- Dahiya R, Keller JD, Litofsky NS, et al. Temporal bone fractures: otic capsule sparing versus otic capsule violating clinical and radiographic considerations. J Trauma 1999;47:1079-83. [Crossref] [PubMed]
- Brodie HA, Thompson TC. Management of complications from 820 temporal bone fractures. Am J Otol 1997;18:188-97. [PubMed]
- Kanona H, Anderson C, Lambert A, et al. A large case series of temporal bone fractures at a UK major trauma centre with an evidence-based management protocol. J Laryngol Otol 2020;134:205-12. [Crossref] [PubMed]
- Ricciardiello F, Mazzone S, Longo G, et al. Our Experience on Temporal Bone Fractures: Retrospective Analysis of 141 Cases. J Clin Med 2021;10:201. [Crossref] [PubMed]
- Yalçıner G, Kutluhan A, Bozdemir K, et al. Temporal bone fractures: evaluation of 77 patients and a management algorithm. Ulus Travma Acil Cerrahi Derg 2012;18:424-8. [Crossref] [PubMed]
- Chang CY, Cass SP. Management of facial nerve injury due to temporal bone trauma. Am J Otol 1999;20:96-114. [PubMed]
- Yadav S, Panda NK, Verma R, et al. Surgery for post-traumatic facial paralysis: are we overdoing it? Eur Arch Otorhinolaryngol 2018;275:2695-703. [Crossref] [PubMed]
- Darrouzet V, Duclos JY, Liguoro D, et al. Management of facial paralysis resulting from temporal bone fractures: Our experience in 115 cases. Otolaryngol Head Neck Surg 2001;125:77-84. [Crossref] [PubMed]
- Yetiser S, Hidir Y, Gonul E. Facial nerve problems and hearing loss in patients with temporal bone fractures: demographic data. J Trauma 2008;65:1314-20. [Crossref] [PubMed]
- McKennan KX, Chole RA. Facial paralysis in temporal bone trauma. Am J Otol 1992;13:167-72. [PubMed]
- Abbaszadeh-Kasbi A, Kouhi A, Ashtiani MTK, et al. Conservative versus Surgical Therapy in Managing Patients with Facial Nerve Palsy Due to the Temporal Bone Fracture. Craniomaxillofac Trauma Reconstr 2019;12:20-6. [Crossref] [PubMed]
- Nash JJ, Friedland DR, Boorsma KJ, et al. Management and outcomes of facial paralysis from intratemporal blunt trauma: a systematic review. Laryngoscope 2010;120:1397-404. [Crossref] [PubMed]
- Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet 2004;364:1321-8. [Crossref] [PubMed]
- Alderson P, Roberts I. Corticosteroids for acute traumatic brain injury. Cochrane Database Syst Rev 2005;CD000196. [PubMed]
- Hato N, Nota J, Hakuba N, et al. Facial nerve decompression surgery in patients with temporal bone trauma: analysis of 66 cases. J Trauma 2011;71:1789-92; discussion 1792-3. [Crossref] [PubMed]
- Xu P, Jin A, Dai B, et al. Surgical timing for facial paralysis after temporal bone trauma. Am J Otolaryngol 2017;38:269-71. [Crossref] [PubMed]
- Quaranta A, Campobasso G, Piazza F, et al. Facial nerve paralysis in temporal bone fractures: outcomes after late decompression surgery. Acta Otolaryngol 2001;121:652-5. [Crossref] [PubMed]
- Fisch U. Management of intratemporal facial nerve injuries. J Laryngol Otol 1980;94:129-34. [Crossref] [PubMed]
- Savva A, Taylor MJ, Beatty CW. Management of cerebrospinal fluid leaks involving the temporal bone: report on 92 patients. Laryngoscope 2003;113:50-6. [Crossref] [PubMed]
- Khan R, Sajjad M, Khan AA, et al. Comparison of Lumbar Drain Insertion And Conservative Management In The Treatment Of Traumatic CSF Rhinorrhoea. J Ayub Med Coll Abbottabad 2019;31:441-4. [PubMed]
- Ratilal BO, Costa J, Pappamikail L, et al. Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures. Cochrane Database Syst Rev 2015;CD004884. [Crossref] [PubMed]
- Brodie HA. Prophylactic antibiotics for posttraumatic cerebrospinal fluid fistulae. A meta-analysis. Arch Otolaryngol Head Neck Surg 1997;123:749-52. [Crossref] [PubMed]
- Villalobos T, Arango C, Kubilis P, et al. Antibiotic prophylaxis after basilar skull fractures: a meta-analysis. Clin Infect Dis 1998;27:364-9. [Crossref] [PubMed]
- Ratilal BO, Costa J, Sampaio C, et al. Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures. Cochrane Database Syst Rev 2011;CD004884. [PubMed]
- Grant JR, Arganbright J, Friedland DR. Outcomes for conservative management of traumatic conductive hearing loss. Otol Neurotol 2008;29:344-9. [Crossref] [PubMed]
- Padmakumar V, Ramesh Kumar E, Ramakrishnan VR. A Prospective Study on Temporal Bone Involvement in Polytrauma Patients and the Effect of Early Diagnosis on Hearing Loss. Indian J Otolaryngol Head Neck Surg 2020;72:30-5. [Crossref] [PubMed]
- Kurihara YY, Fujikawa A, Tachizawa N, et al. Temporal Bone Trauma: Typical CT and MRI Appearances and Important Points for Evaluation. Radiographics 2020;40:1148-62. [Crossref] [PubMed]
- Dempewolf R, Gubbels S, Hansen MR. Acute radiographic workup of blunt temporal bone trauma: maxillofacial versus temporal bone CT. Laryngoscope 2009;119:442-8. [Crossref] [PubMed]
- Szczupak M, Kravietz A, Patel J, et al. Utilization of Computed Tomography in Temporal Bone Fractures at a Large Level I Trauma Center. Laryngoscope 2021;131:E278-82. [Crossref] [PubMed]
Cite this article as: Green L, Wang J, Li C, Tully D, Woliansky J, Gumm K, Martin K, Read D, Iseli C. Management of petrous temporal bone fractures: a 5-year experience from an Australian major trauma centre. Aust J Otolaryngol 2022;5:27.


