Grafts in septorhinoplasty: a systematic review and future directions
Introduction
The presence or treatment of congenital, traumatic, and oncological defects of the nose can compromise nasal airway function and cosmesis. Congenital and traumatic septal deviation is present in up to 80% of the general population (1), and facial skin cancer most commonly affects the nose (1). A myriad of reconstructive surgical procedures has been described to address congenital and acquired nasal deformity, ranging from simple nasal bone fracture reduction, skin graft and local flaps to septorhinoplasty and complex microsurgical composite reconstruction. Septorhinoplasty is the third most common procedure in the United States, accounting for over $1.1 billion in 2016 alone (2).
Nasal reconstruction poses unique challenges for a variety of reasons in addition to the obvious cosmetic ramifications. The nose is a tri-lamellar structure that is difficult to duplicate synthetically. The external skin-soft tissue envelope protecting the internal nasal structures can be thin, and is in close proximity with the nasal mucosa which commonly harbors pathogens (3). The outcome of any reconstructive procedure must withstand the normal aerodynamic forces of airflow during respiration that filters and humidifies air, in addition to air expulsion during sneezing (1).
Commonly, the native nasal septum alone is sufficient as an autologous cartilage graft donor site for addition of structure to the nasal skeleton and volume to the nose. However, when there is insufficient septal cartilage for harvest, alternative grafts are necessary. Current alternative grafts to native septal cartilage are either biologic or synthetic/alloplastic (4). Biologic grafts are predominantly autologous (i.e., autograft), and to a much lesser extent allogeneic (i.e., allograft). Autografts are harvested from one anatomical location and used elsewhere on the same individual, making the patient both donor and recipient. Traditionally, the autologous costal cartilage (ACC) graft is the gold standard when an individual’s nasal cartilage is insufficient during septorhinoplasty. An alternative is the auricular cartilage; however it is curved, thin, and scarce (1,4). Allografts, derived from another within the same species, are commonly cadaveric irradiated homologous costal cartilage (IHCC) grafts. The most common commercially available alloplastic grafts are Medpor (porous high-density polyethylene), Gore-Tex (expanded polytetrafluoroethylene), and Silicone.
The literature describes the ideal graft in septorhinoplasty on microscopic and macroscopic levels (1,4). Biocompatibility, bioactivity, and biodegradability are essential for graft success on a microscopic level. Biocompatibility is influenced by the immunogenicity of a graft (1). Grafts with low immunogenicity elicit lesser host immune responses, resulting in greater biocompatibility and generally lower infection rates. Bioactive factors promote integration by inducing chondrogenesis and/or osteogenesis without fibrous tissue encapsulation sealing the implant off (5,6). Biodegradation ideally occurs at a balanced rate whereby the graft maintains its properties and mechanical strength for an adequate time to allow sufficient host response to form the desired tissue before degrading into biocompatible waste products (6). Whilst greater porosity influences cellular migration, ingrowth and vascularization, excessive pore size hinders strength (6). Generally, porosity above 50 µm is recommended as many studies have shown that greater porosity is associated with increasing cellular concentration, DNA content, glycosaminoglycan (GAG) and collagen synthesis (7).
Macroscopically, grafts that mimic the biomechanical and functional properties of the target tissue without being a vector for disease are ideal (4). In addition to maintaining volume without extruding over time, grafts should resist external and internal physiological forces. Ideal grafts are expediently produced or procured inexpensively on-demand to patient-specific requirements, or are intra-operatively customizable, and are not associated with donor-site morbidity. Infusing grafts with antimicrobial agents is preferred in limiting perioperative infection.
Considering how common septorhinoplasty is, many studies have analyzed complication rates of biologic and alloplastic grafts resulting in multiple systematic reviews with varying results. This systematic review aims to summarize the complication rates of commonly used septorhinoplasty grafts from systematic reviews and provide an overview of emerging alternatives. We present the following article in accordance with the PRISMA reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-22-12/rc) (8).
Methods
Eligibility criteria
Systematic reviews with or without meta-analysis including more than 10 primary studies were included whilst narrative reviews, case reports, and abstracts were excluded. Furthermore, reviews without pooled analysis of complication rates, not full text, not written in English, or not pertaining to humans were excluded.
Information sources
Two independent reviewers (BSN and AEY) conducted a systematic literature review of four databases [Medline, EMBASE, Google Scholar and Evidence-Based Medicine Reviews (EMBR)] up to the 8th of November 2021 using the below search strategy. References of retrieved articles were also manually searched for additional articles missed in the original literature review.
Search methods
A search strategy was generated using the PICO structure: Population—patients receiving septorhinoplasty for functional or aesthetic reasons; Intervention—ACC grafts in septorhinoplasty; Comparison—allogeneic or alloplastic grafts in septorhinoplasty; Outcome—complication rates post-operatively. The following search strategy was used “(nasal augmentation or nasal reconstruction or septoplasty or rhinoplasty or septorhinoplasty) AND ([rib graft or autologous costal cartilage graft or cadaveric cartilage graft] OR [alloplastic implant or silicone or Med*por or Gore*Tex])”. Two independent reviewers (BSN and AEY) selected studies against the pre-defined inclusion criteria and any disagreements were resolved by discussion.
Data collection and analysis
Two independent reviewers (BSN and AEY) extracted data regarding study characteristics, complication rates and required data to complete a Risk of Bias in Systematic Reviews (ROBIS) assessment. Any disagreements were resolved by discussion between reviewers and, where required, with senior authors (PM and SC). Data extracted and tabulated is as follows:
- Study characteristics included study design, implants (i.e., interventions) analyzed, level of evidence and included study types, number of included studies and their year range, number of included patients, degree of overlap of included primary studies, length of follow-up, databases searched, and risk of bias assessment tool used;
- Complications included overall complication rate, infection, warping, resorption, extrusion, hypertrophic scarring, pneumothorax, and revision and removal surgery.
The degree of overlap of primary studies between included systematic reviews was calculated using the Corrected Covered Area (CCA) method by two independent reviewers (BSN and AEY) (9). Considering the degree of overlap of primary studies and heterogeneity of included systematic reviews, results of complication rates were described narratively as further meta-analysis was not feasible (10).
Results
Search results
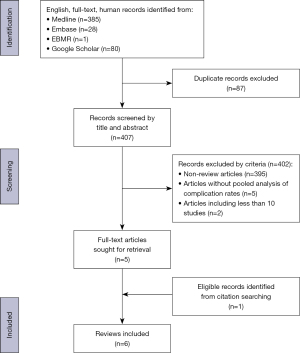
The initial literature search yielded 494 articles of which 87 were excluded as duplicates. Four hundred and seven unique titles and abstracts were then screened using pre-defined inclusion and exclusion criteria yielding five systematic reviews, which were included. Two systematic reviews were excluded in this process as they included 10 or less primary studies and thus did not meet inclusion criteria (11,12). Further manual searching of the citations of included reviews resulted in one further systematic review being included (Figure 1) (8,13). The six included systematic reviews were published between 2008 and 2020 and analyzed ACC with alloplastic grafts (n=2) (2,14), ACC with IHCC grafts (n=1) (15), alloplastic grafts only (n=2) (16,17), and ACC grafts only (n=1) (13) (Table 1).
Table 1
| Author, year | Implant used | Study design | Level of evidence | No. of studies (year) | No. of patients | Overlap (overlap/total No. of studies) | Follow up | Databases searched | Risk of bias assessment tool |
|---|---|---|---|---|---|---|---|---|---|
| Peled et al., 2008 (17) | Silicone; Medpor; Gore-Tex | Meta-analysis & Systematic Review | IV | 20 (1966–2005) | 4,264 | 35% (7/20) | 4 weeks–11 years | Medline | N/A |
| Hoang et al., 2018 (16) | Silicone; Gore-Tex | Meta-analysis & Systematic Review | IV | 18 (1988–2016) | 7,759 | 66.7% (12/18) | 6 days–17 years | Medline, PubMed, Cochrane | N/A |
| Varadharajan et al., 2014 (13) | ACC† | Systematic Review | IV | 21 (1980–2014) | 1,545 | 52.4% (11/21) | 3–73 months | Medline, PubMed, Cochrane, EMBASE | ASPS critical appraisal checklist |
| Hudise et al., 2020 (14) | ACC; Silicone; Medpor; Gore-Tex | Meta-analysis & Systematic Review | IV | 30 (1999–2018) | 1,013 | 76.7% (23/30) | 2.5–106 months | PubMed, Cochrane Library, Web of Science, Virtual Health Library, Google Scholar, SCOPUS | ROBINS-1 |
| Liang et al., 2018 (2) | ACC; Silicone; edpor; Gore-Tex | Meta-analysis & Systematic Review | IV | 53 (1973–2017) | 5,415 | 67.9% (36/53) | 3–72 months | EMBASE, PubMed | MINORS |
| Vila et al., 2020 (15) | ACC; IHCC‡ | Meta-analysis & Systematic Review | IV | 28 (1990–2017) | 1,041 | 50% (14/28) | 6–48 months | Medline, EMBASE, Scopus, CENTRAL, ClinicalTrials.gov | Cochrane Collaboration Risk of Bias Tool |
†, Autologous Costal Cartilage; ‡, Irradiated Homologous Costal Cartilage. N/A, not applicable; ACC, autologous costal cartilage; ASPS, American Society of Plastic Surgeons; ROBINS-1, Risk of Bias In Non-randomized Studies; MINORS, Methodological Index for Non-Randomized Studies; IHCC, irradiated homologous costal cartilage.
Quality of included systematic reviews
In total, 170 primary studies were cited in the six systematic reviews with 111 of these being unique primary studies yielding a CCA of 10.63% which is considered moderate to high overlap (9). Table 2 is a citation matrix representing the number of overlapping primary studies between any two given systematic reviews. Overall, Liang et al. had the most overlap of included primary studies with all other reviews (50 citations), however, Hudise et al. had the highest degree of overlap of any one systematic review (76.7%) (Table 1) (2,14).
Table 2
| Review | Peled (17) | Hoang (16) | Varadharajan (13) | Hudise (14) | Liang (2) | Vila (15) |
|---|---|---|---|---|---|---|
| Peled (17) | N/A | 5 | 0 | 1 | 3 | 0 |
| Hoang (16) | N/A | N/A | 0 | 0 | 8 | 0 |
| Varadharajan (13) | N/A | N/A | N/A | 1 | 7 | 5 |
| Hudise (14) | N/A | N/A | N/A | N/A | 21 | 5 |
| Liang (2) | N/A | N/A | N/A | N/A | N/A | 11 |
| Vila (15) | N/A | N/A | N/A | N/A | N/A | N/A |
Numbers in each cell represent the total number of overlapping primary studies between any two systematic reviews. N/A, not applicable.
Using the ROBIS tool, two reviews were considered to have a high overall risk of bias representing 30% of the included reviews (16-18). The most common domain introducing bias was in synthesis and findings (50%) (Table 3). Peled et al. did not have pre-defined eligibility criteria, searched a single database, did not use a standardized risk of bias assessment tool, and did not employ funnel plots or sensitivity analysis in the synthesis of their results (17). Similarly, Hoang et al. did not employ a formal assessment of risk of bias or heterogeneity (16).
Table 3
| Review | Phase 2 | Phase 3 | ||||
|---|---|---|---|---|---|---|
| Study eligibility criteria | Identification and selection of studies | Data collection and study appraisal | Synthesis and findings | Risk of bias in the review | ||
| Peled (17) | ☹ | ☹ | ☹ | ☹ | ☹ | |
| Hoang (16) | ☹ | ☺ | ☹ | ☹ | ☹ | |
| Varadharajan (13) | ☺ | ☺ | ☺ | ☹ | ☺ | |
| Hudise (14) | ☹ | ☺ | ☺ | ☺ | ☺ | |
| Liang (2) | ☺ | ☺ | ☺ | ☺ | ☺ | |
| Vila (15) | ☺ | ☺ | ☺ | ☺ | ☺ | |
☺ = low risk; ☹ = high risk.
Qualitative synthesis
Complications associated with graft use were thematically organized: overall complication rate, infection, warping, resorption, extrusion, hypertrophic scarring, pneumothorax, and revision and removal surgery. Results of complication rates from each systematic review for ACC, IHCC, and alloplastic grafts are listed in Table 4.
Table 4
| ACC | IHCC | Alloplastic | ||||||
|---|---|---|---|---|---|---|---|---|
| % of donor site [CI] | % of recipient site [CI] | % of combined sites [CI] | % of silicone [CI] | % of Med-por [CI] | % of Gore-Tex [CI] | % of combined alloplastics [CI] | ||
| Overall complication | 3.2 (13), 7 [4–11.6] (2) | 11.4 (13), 14 [10–19] (2) | 6.9 [4.3–9.6] (14) | NS | 9.2 (16) | NS | 5.3 (16) | 7.8 [4–11.5] (14), 8 [5–11] (2) |
| Infection | 0.6 (13) | 2.5 (13) | 2 [0–5] (15), 2.1 [1–3.3] (14), 6.8 [4.7–9.7] (2) | 3 [1–8] (15) | 1.9 (16), 2.1 [0.6–7.3] (2), 3.7 (17) | 2.3 [1–8] (2), 6 (17) | 1.3 [0.6–3] (2), 1.4 (16), 4 (17) | 3.7 [0.4–7] (14), 4.6 [0.3–44.8] (2) |
| Warping | N/A | 1.5 [0.3–2.8] (14), 5.2 (13), 5.6 [4–7.7] (2), 6 [2–11] (15) | N/A | 5 [1–12] (15) | 5.1 [2–11.6] (2) | NS | 0.9 [0.6–1.7] (2) | 1.6 [0.5–3.7] (14) |
| Resorption | N/A | 0.9 (13), 1 [0–2] (15), 3.9 [2.6–5.8] (2), 4.1 [2.5–5.6] (14) | N/A | 4 [0–13] (15) | 1.9 [0.1–24] (2) | 2.5 [0.2–30] (2) | 0.7 [0.1–3.6] (2) | NS |
| Extrusion | N/A | 0.6 (13), 1.5 [0.6–3.8] (2) | N/A | NS | 0.7 (16), 1.6 [0.5–5.2] (2), 3.2 (17) | 2 (17), 5.1 [2.5–10] (2) | 1.1 (17), 1.2 (16), 2.9 [2.2–4] (2) | 1.1 [0.2–5.2] (2) |
| Hypertrophic scar | 2.9 (13), 3.8 [1.7–8] (2) | NS | 1.8 [0.2–3.4] (14) | NS | NS | NS | NS | 1.5 [0.4–3.5] (14) |
| Pneumothorax | 0.1 (13), 3.1 [1.5–6.5] (2) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Revision surgery | N/A | 4.2 [2.4–6] (14), 5 [1–10] (15), 5.4 (13), 8 [4.8–12.9] (2) | N/A | 7 [3–12] (15) | 3.9 [1–14.2] (2), 6.8 (16) | 5.7 [2.8–11.3] (2) | 2 [0.3–14] (2), 2.4 (16) | 5 [1.8–13] (2), 3.7 [1.0–6.4] (14) |
| Removal surgery | N/A | 1.9 [1–3.6] (2) | N/A | NS | 6.5 (17), 12 [6.5–19.9] (2) | 3.1 (17), 4.5 [2–10] (2) | 3.1 (17), 3.6 [2–6.2] (2) | 2.2 [0.4–10.5] (2) |
CI, confidence interval; ACC, autologous costal cartilage; IHCC, irradiated homologous costal cartilage; NS, not stated; N/A, not applicable.
Overall complication rate
Overall complication rates at the recipient site for ACC grafts were 11.4% (13) and 14% (2), and at the donor site were 3.2% (13) and 7% (2). One systematic review (14) reported overall complication rates irrespective of donor or recipient site as 6.9% for ACC grafts. Overall complication rates for alloplastic grafts were found to be 8% (2) and 7.8% (14). No systematic review provided overall complication rates for IHCC grafts.
Infection
All six systematic reviews (2,13-17) analyzed infection rates by graft type. Gore-Tex was found to have varying infection rates of 1.3% (2), 1.4% (16), and 4% (17). Medpor demonstrated rates of 2.3% (2) and 6% (17). Silicone had reported rates of 1.9% (16), 2.1% (2), and 3.7% (17). Combined alloplastic graft infection rates were 3.7% (14) and 4.6% (2). ACC grafts were found to cause infection at the donor site in 0.6% (13) of cases, and at the recipient site at rates of 2% (15), 2.1% (14), 2.5% (13) and 6.8% (2). IHCC grafts demonstrated an infection rate of 3% (15).
Warping and resorption
Of the four systematic reviews (2,13-15) reporting rates of warping amongst ACC grafts, only two (2,14) documented comparative alloplastic warping rates. One systematic review found the combined alloplastic warping rate as 1.6% (14), whilst another differentiated warping rates by alloplastic with Silicone having the highest warping rate at 5.1%, Gore-Tex the lowest at 0.9%, and no statistically significant data for Medpor (2). Warping was generally higher with ACC grafts [5.2% (13), 5.6% (2), and 6% (15)], however one systematic review reported rates as low as 1.5% (14).
One systematic review comparing ACC and alloplastic resorption rates found higher resorption rates for ACC grafts (3.9%) compared with Silicone (1.9%), Medpor (2.5%), and Gore-Tex (0.69%) (2). Contrastingly, three systematic reviews that analyzed resorption rates for ACC grafts found varied rates [0.9% (13), 1% (15), and 4.1% (14)]. IHCC graft warping and resorption rates were 5% and 4% respectively (15).
Extrusion
Three systematic reviews (2,16,17) documented extrusion rates. ACC grafts generally extruded at lower rates [0.6% (13) and 1.5% (2)]. Medpor had higher rates of extrusion [2% (17) and 5.1% (2)] than Gore-Tex [1.1% (17), 1.2% (16), and 2.9% (2)]. Silicone had varying rates of extrusion at 0.7% (16), 1.6% (2), and 3.2% (17).
Hypertrophic scarring and pneumothorax
Three systematic reviews (2,13,14) reported hypertrophic scarring rates which were generally higher for ACC [1.8% (14), 2.9% (13), and 3.8% (2)] than alloplastic grafts [1.5% (14)]. Pneumothorax was solely associated with ACC grafts, with varying rates between 0.1% (13) and 3.1% (2).
Revision and removal surgery
Six systematic reviews (2,13-17) analyzed alloplastic, IHCC and ACC grafts with regards to rates of surgical revision or removal. One systematic review showed ACC grafts had lower removal rates (1.9%) and higher revision rates (8%) (2). Contrastingly, in the same study alloplastics generally had higher removal than revision rates: Silicone’s removal and revision rate was 12% and 3.9% respectively, Medpor 4.5% and 5.7%, and Gore-Tex 3.6% and 2% (2). Another systematic review showed similar revision surgery rates for alloplastic (3.7%) and ACC (4.2%) grafts (14). IHCC grafts had higher revision rates [7% (15)] compared to ACC grafts [5% (15)] which was also reflected in another systematic review where the ACC revision rate was 5.4% (13). One systematic review found removal rates to be 6.5% for Silicone, and 3.1% for Medpor and Goretex (17). Another systematic review demonstrated revision rates of 6.8% for Silicone, and 2.4% for Gore-Tex (16).
Discussion
This review identified six systematic reviews with or without meta-analysis that show neither ACC, allogeneic, or alloplastic grafts are superior in every clinical situation during septorhinoplasty. Warping, resorption, revision surgery and donor-site morbidity were generally higher with ACC graft use. Infection, extrusion, and removal surgery were generally higher amongst all alloplastic grafts compared to ACC grafts. However, there was variation of these rates between different types of alloplastic grafts. IHCC grafts had similar complication rates to ACC grafts with regards to infection, warping, resorption, and secondary surgery when used as dorsal onlay grafts only.
Biologic grafts
ACC grafts are popular for their intra-operative versatility and low infection risk. In addition to being relatively abundant in supply, ACC grafts are sufficiently rigid to provide structural support, or be morselized as a filler (4). Being autologous, ACC grafts have inherently low immunogenicity and thus low infection rates (1,15). When an infection is present, intravenous antibiotics have greater penetrance in ACC grafts compared with synthetic alternatives, thus reducing requirements for secondary surgery for ACC grafts due to infection (12). Despite its popularity, there are multiple challenges with ACC grafts; donor-site morbidity, longer operating times for harvest, revision surgery and warping (13).
IHCC grafts are a potential solution for the drawbacks of ACC grafts as they have low infection rates, no donor-site morbidity, and are readily available eliminating long operating times. Although IHCC grafts are irradiated to lower infection risk, this process causes decellularization limiting in vivo remodeling thereby promoting greater resorption and revision surgery rates (1). Despite this, a recent meta-analysis by Vila et al. demonstrated similar rates of warping between IHCC and ACC grafts (15). However, their study only included IHCC dorsal onlay grafts where structural support was not required (15). Traditionally, IHCC grafts are limited to situations where structural support isn’t crucial to compensate for their varying warping rates and poorer retention (15,19). Considering this, IHCC grafts are generally less popular than ACC grafts, contributing to a paucity of long-term follow-up studies (19).
Alloplastic grafts
Another alternative to ACC grafts is the alloplastic graft. Alloplastics can be cost-effectively produced providing an abundance of supply without donor-site morbidity. Whilst some alloplastics can be intra-operatively carved to specific dimensions, others can be pre-fabricated to generic shapes such as alar battens or L-struts (4). However, there is no readily available alloplastic graft suitable in every septorhinoplasty operation (4) and no studies to date have quantitatively compared the biomechanical properties of alloplastics with septal or costal cartilage (1,20). Despite this, they have been used extensively in select groups (21).
Of the three common alloplastics, the softness of Gore-Tex makes it appropriate for dorsal onlay grafting but too weak for structural support (4). With intermediate porosity ranging from 10–30 µm (21), Gore-Tex allows limited tissue ingrowth for stabilization (4). Medpor possesses the highest porosity of the three alloplastics [125–250 µm (21)], promoting significant tissue ingrowth. Combined with its superior rigidity, this makes Medpor suitable for structural support (4). However, this also contributes to Medpor’s extrusion and removal rates, with the removal operation being more difficult considering the tissue ingrowth (2). Silicone is cheap and easily carved intra-operatively, promoting its use in dorsal onlay techniques (2,4). As silicone is non-porous, there is significant fibrous encapsulation and subsequent calcification over time promoting the highest complication and removal surgery rates of the three alloplastics (2). Interestingly however, the rates of silicone extrusion and displacement amongst Asians are lower compared with Caucasians. Asian patients have a thicker skin-soft tissue envelope that likely creates a more forgiving environment for silicone implants compared with Caucasian patients (4). Consequently, silicone is more popular with Asian surgeons, resulting in greater experience and refined surgical techniques (22).
In comparing alloplastic and ACC grafts, Liang et al. demonstrated higher removal rates for alloplastics compared with higher revision rates for ACC grafts (2). This is likely attributable to ACC grafts warping at higher rates but being autologous in nature, often only requiring revision rather than removal. Contrastingly, alloplastic grafts extrude at higher rates and are less adept to manipulation for revision, therefore requiring removal.
Limitations
The major limitation of this systematic review is the heterogeneity of included systematic reviews and significant overlap of included primary studies rendering meta-analysis or comparison of results between studies unfeasible (9,23). Furthermore, all reviews relied heavily on retrospective studies making their level of evidence IV at best. Two of the included systematic reviews were also considered to have a high risk of bias which limits interpretation of their results.
Future directions
Tissue-engineered grafts in septorhinoplasty have emerged in the hope of eliminating the drawbacks of donor-site morbidity and longer operating times associated with biologic grafts whilst retaining their inherently low immunogenicity and tissue versatility. A tissue-engineered graft aims to produce biomimetic tissue by utilizing the optimal combination of a cellular source, stimuli, and scaffolding (24).
Cellular source in tissue-engineering neo-cartilage is primarily either chondrocytes or mesenchymal stem cells (MSCs) (1,24). Although chondrocytes harvested from bovine (25), rabbit (26), and ovine (27) sources have been investigated, only autologous sources have been approved for human use and thus the issue of donor-site morbidity persists (28). Autologous or allogeneic MSCs can grow rapidly but produce fibrocartilage that lacks the loading and stretching properties that hyaline septal cartilage possesses (24). Genetic modifications present a future avenue for research to obtain appropriate hyaline cartilaginous tissue from MSCs (29,30).
Stimulation whilst culturing chondrocytes through either 3D culture systems (31), growth factors (32), or MSCs (24) is important as chondrocytes dedifferentiate to adopt fibroblastic characteristics after three monolayer culture passages (24). To combat the significant quantity of chondrocytes required to engineer the volume of cartilage required in septorhinoplasty, methods such as incorporating autologous MSCs (25) and using photocrosslinkable bioinks that can bioprint chondrocytes instead of seeding them have been studied (33). However, further research is required to overcome issues of time and cost to allow production at scale to be viable.
Clinically, tissue-engineered grafts have demonstrated promising but limited results (1). There are no systematic reviews, meta-analyses, large animal studies, or standardized animal models for tissue-engineered grafts in septorhinoplasty (1,30-32,34). Only five uncontrolled cohort studies in small human populations have been conducted to date without any report of significant adverse events (35-39).
Bioactive-biocompatible polymer hybrid scaffolds combine porous biodegradable biocompatible polymeric scaffolds with bioactive materials to improve integration and reduce extrusion rates when compared to alloplastics (40-42). Whilst no hybrid scaffolds have been approved in septorhinoplasty, the success of hybrid scaffolds like Chondrotissue® (Biotissue) in replacing articular cartilage is promising (43,44). Wu et al. have demonstrated in vivo that the combination of a bioactive ceramic silica-based glass (Bioglass®) with a polymer scaffold exhibited greater hydrophilicity, adherence, and stimulated thicker cartilage-like tissue with better biomechanics than Bioglass® alone (41). Yao et al. also demonstrated a hybrid Bioglass®-polymer scaffold had degradation rates and mechanical strength superior to Bioglass® alone (42). Characterizing the ability of Bioglass®-polymer scaffold composites to stimulate chondrogenesis in vivo requires further research.
Conclusions
Our systematic review of six systematic reviews suggests alloplastic, ACC and IHCC grafts are viable alternatives when native septal cartilage is insufficient during septorhinoplasty. When selecting grafts, surgeons should consider the purpose and complications associated with each graft and educate patients appropriately. Whilst ACC grafts remain the preferred alternative, it is evident that the ‘ideal’ graft has yet to be achieved. Tissue-engineered grafts and bioactive-biocompatible polymer composites hold promise but require further research to overcome their limitations and nascence.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-22-12/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-22-12/coif). PM serves as an unpaid editorial board member of Australian Journal of Otolaryngology from January 2019 to December 2022.The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lavernia L, Brown WE, Wong BJF, et al. Toward tissue-engineering of nasal cartilages. Acta Biomater 2019;88:42-56. [Crossref] [PubMed]
- Liang X, Wang K, Malay S, et al. A systematic review and meta-analysis of comparison between autologous costal cartilage and alloplastic materials in rhinoplasty. J Plast Reconstr Aesthet Surg 2018;71:1164-73. [Crossref] [PubMed]
- Karnes J, Salisbury M, Schaeferle M, et al. Porous High-density Polyethylene Implants (Medpor) for Nasal Dorsum Augmentation. Aesthetic Surgery Journal 2000;20:26-30. [Crossref]
- Ansari K, Asaria J, Hilger P, et al. Grafts and implants in rhinoplasty—Techniques and long-term results. Operative Techniques in Otolaryngology-Head and Neck Surgery 2008;19:42-58. [Crossref]
- Asselin A, Hattar S, Oboeuf M, et al. The modulation of tissue-specific gene expression in rat nasal chondrocyte cultures by bioactive glasses. Biomaterials 2004;25:5621-30. [Crossref] [PubMed]
- Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater 2013;9:4457-86. [Crossref] [PubMed]
- Nava MM, Draghi L, Giordano C, et al. The effect of scaffold pore size in cartilage tissue engineering. J Appl Biomater Funct Mater 2016;14:e223-9. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Pieper D, Antoine SL, Mathes T, et al. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol 2014;67:368-75. [Crossref] [PubMed]
- Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, 2019.
- Alhussain OH, Alhussain GO, Rajkhan AA, et al. Efficacy of autologous vs. homologous costal cartilage grafts in dorsal augmentation rhinoplasty: a systematic review and meta-analysis. Ann Med Health Sci Res 2020;10:1100-4.
- Wee JH, Park MH, Oh S, et al. Complications associated with autologous rib cartilage use in rhinoplasty: a meta-analysis. JAMA Facial Plast Surg 2015;17:49-55. [Crossref] [PubMed]
- Varadharajan K, Sethukumar P, Anwar M, et al. Complications Associated With the Use of Autologous Costal Cartilage in Rhinoplasty: A Systematic Review. Aesthet Surg J 2015;35:644-52. [Crossref] [PubMed]
- Hudise JY, Aldhabaan SA, Aldosari BF. Complications of the nasal dorsum reconstruction using autologous or alloplastic grafts: evidence from systematic review and meta-analysis. Braz J Otorhinolaryngol 2022;88:406-20. [Crossref] [PubMed]
- Vila PM, Jeanpierre LM, Rizzi CJ, et al. Comparison of Autologous vs Homologous Costal Cartilage Grafts in Dorsal Augmentation Rhinoplasty: A Systematic Review and Meta-analysis. JAMA Otolaryngol Head Neck Surg 2020;146:347-54. [Crossref] [PubMed]
- Hoang HLT, Januszyk M, Boyd JB. A systematic review of complications associated with nasal augmentation implants: expanded polytetrafluoroethylene (Gore-Tex) versus silicone. Journal of Cosmetic Medicine 2018;2:27-31. [Crossref]
- Peled ZM, Warren AG, Johnston P, et al. The use of alloplastic materials in rhinoplasty surgery: a meta-analysis. Plast Reconstr Surg 2008;121:85e-92e. [Crossref] [PubMed]
- Whiting P, Savović J, Higgins JP, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016;69:225-34. [Crossref] [PubMed]
- Wee JH, Mun SJ, Na WS, et al. Autologous vs Irradiated Homologous Costal Cartilage as Graft Material in Rhinoplasty. JAMA Facial Plast Surg 2017;19:183-8. [Crossref] [PubMed]
- Griffin MF, Premakumar Y, Seifalian AM, et al. Biomechanical characterisation of the human nasal cartilages; implications for tissue engineering. J Mater Sci Mater Med 2016;27:11. [Crossref] [PubMed]
- Mella J, Christophel J, Park S. Are Alloplastic Implants Safe in Rhinoplasty? Laryngoscope 2020;130:1854-6. [Crossref] [PubMed]
- Shirakabe Y, Suzuki Y, Lam SM. A systematic approach to rhinoplasty of the Japanese nose: a thirty-year experience. Aesthetic Plast Surg 2003;27:221-31. [Crossref] [PubMed]
- Glenny AM, Altman DG, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005;9:1-134. iii-iv. [Crossref] [PubMed]
- Kim MS, Kim HK, Kim DW. Cartilage tissue engineering for craniofacial reconstruction. Arch Plast Surg 2020;47:392-403. [Crossref] [PubMed]
- Pleumeekers MM, Nimeskern L, Koevoet WLM, et al. Cartilage Regeneration in the Head and Neck Area: Combination of Ear or Nasal Chondrocytes and Mesenchymal Stem Cells Improves Cartilage Production. Plast Reconstr Surg 2015;136:762e-74e. [Crossref] [PubMed]
- Kushnaryov A, Yamaguchi T, Briggs KK, et al. Evaluation of Autogenous Engineered Septal Cartilage Grafts in Rabbits- A Minimally Invasive Preclinical Model. Adv Otolaryngol 2014;2014:415821. [Crossref] [PubMed]
- Oseni AO, Butler PE, Seifalian AM. Optimization of chondrocyte isolation and characterization for large-scale cartilage tissue engineering. J Surg Res 2013;181:41-8. [Crossref] [PubMed]
- Phull AR, Eo SH, Abbas Q, et al. Applications of Chondrocyte-Based Cartilage Engineering: An Overview. Biomed Res Int 2016;2016:1879837. [Crossref] [PubMed]
- Lee WY, Wang B. Cartilage repair by mesenchymal stem cells: Clinical trial update and perspectives. J Orthop Translat 2017;9:76-88. [Crossref] [PubMed]
- Tan AR, Hung CT. Concise Review: Mesenchymal Stem Cells for Functional Cartilage Tissue Engineering: Taking Cues from Chondrocyte-Based Constructs. Stem Cells Transl Med 2017;6:1295-303. [Crossref] [PubMed]
- Caron MM, Emans PJ, Coolsen MM, et al. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage 2012;20:1170-8. [Crossref] [PubMed]
- Alexander TH, Sage AB, Chen AC, et al. Insulin-like growth factor-I and growth differentiation factor-5 promote the formation of tissue-engineered human nasal septal cartilage. Tissue Eng Part C Methods 2010;16:1213-21. [Crossref] [PubMed]
- Zhang YS, Yue K, Aleman J, et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann Biomed Eng 2017;45:148-63. [Crossref] [PubMed]
- Di Gesù R, Acharya AP, Jacobs I, et al. 3D printing for tissue engineering in otolaryngology. Connect Tissue Res 2020;61:117-36. [Crossref] [PubMed]
- Ceccarelli G, Gentile P, Marcarelli M, et al. In Vitro and In Vivo Studies of Alar-Nasal Cartilage Using Autologous Micro-Grafts: The Use of the Rigenera® Protocol in the Treatment of an Osteochondral Lesion of the Nose. Pharmaceuticals (Basel) 2017;10:53. [Crossref] [PubMed]
- Fulco I, Miot S, Haug MD, et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet 2014;384:337-46. [Crossref] [PubMed]
- Gentile P, Scioli MG, Bielli A, et al. Reconstruction of Alar Nasal Cartilage Defects Using a Tissue Engineering Technique Based on a Combined Use of Autologous Chondrocyte Micrografts and Platelet-rich Plasma: Preliminary Clinical and Instrumental Evaluation. Plast Reconstr Surg Glob Open 2016;4:e1027. [Crossref] [PubMed]
- Hoshi K, Fujihara Y, Saijo H, et al. Implant-type Tissue-engineered Cartilage for Secondary Correction of Cleft Lip-nose Patients: An Exploratory First-in-human Trial. Journal of Clinical Trials 2017;7:1-9. [Crossref]
- Yanaga H. Imai K, Yanaga K. Generative surgery of cultured autologous auricular chondrocytes for nasal augmentation. Aesthetic Plast Surg 2009;33:795-802. [Crossref] [PubMed]
- Deliormanlı AM, Atmaca H. Biological Response of Osteoblastic and Chondrogenic Cells to Graphene-Containing PCL/Bioactive Glass Bilayered Scaffolds for Osteochondral Tissue Engineering Applications. Appl Biochem Biotechnol 2018;186:972-89. [Crossref] [PubMed]
- Wu J, Xue K, Li H, et al. Improvement of PHBV scaffolds with bioglass for cartilage tissue engineering. PLoS One 2013;8:e71563. [Crossref] [PubMed]
- Yao Q, Nooeaid P, Detsch R, et al. Bioglass®/chitosan-polycaprolactone bilayered composite scaffolds intended for osteochondral tissue engineering. J Biomed Mater Res A 2014;102:4510-8. [Crossref] [PubMed]
- Siclari A, Mascaro G, Kaps C, et al. A 5-year follow-up after cartilage repair in the knee using a platelet-rich plasma-immersed polymer-based implant. Open Orthop J 2014;8:346-54. [Crossref] [PubMed]
- Wasyłeczko M, Sikorska W, Chwojnowski A. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes (Basel) 2020;10:348. [Crossref] [PubMed]
Cite this article as: Nandakumar BS, Mukherjee P, Yung AE, Nirmalananda A, Ch’ng S. Grafts in septorhinoplasty: a systematic review and future directions. Aust J Otolaryngol 2022;5:28.