
Sinus mucosal thickening on magnetic resonance imaging and inflammation
Introduction
Thickening of mucosa within the paranasal sinuses is frequently detected on diagnostic imaging of the head, even in patients with no apparent rhinologic disease. Incidental mucosal thickening is present in 12–38% of sinus computed tomography (CT) scans in asymptomatic adults (1-3). Magnetic resonance imaging (MRI) may detect even more incidental mucosal changes, with one large scale analysis reporting mucosal thickening in 49% of the general population (4). Previous studies have suggested that mucosal thickening is poorly correlated with sinonasal inflammation, in patients without chronic rhinosinusitis (CRS) (5-8). However, as the paranasal sinuses are only endoscopically accessible in the post-surgical setting, these studies have been unable to correlate imaging with direct endoscopic assessment of the sinuses and have relied upon patient reported symptoms to assess inflammation. In this context, patients who have received surgery for paranasal sinus or skull base tumors provide a convenient population, without CRS, in whom inflammation can be verified endoscopically.
This study aimed to determine the diagnostic performance of sinus MRI mucosal thickening, in patients without CRS, using validated endoscopic examination and patient reported symptoms. We present the following article in accordance with the STARD reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-22-9/rc).
Methods
A cross-sectional diagnostic study was conducted, including patients recruited from a tertiary rhinology practice in Sydney, Australia who underwent paranasal sinus or skull base tumor resection. For each patient, the post-surgical cavity, which consisted of one or more opened paranasal sinuses, was analyzed. Follow-up was performed 3 months after surgery and included an MRI, endoscopy of the post-operative resection cavity, and patient reported outcome measurement through the Sino-Nasal Outcome Test 22 (SNOT-22). Data collection was planned and completed in a retrospective manner—after follow-up for included patients. The study was conducted in accordance with the declaration of Helsinki (as revised in 2013). The study was approved by the St Vincent’s Hospital Human Research Ethics Committee (SVH HREC 09/083). All participants provided informed and written consent for their use of their medical data for research.
Population
Consecutive patients were recruited if they had received a paranasal sinus or skull base tumor resection, with at least 3 months of follow-up. Patients were excluded if they had a history of CRS, as defined by the 2012 EPOS guidelines, or previous endoscopic sinus surgery (ESS) (9). Atopy status was determined via immunoassay testing of blood samples taken perioperatively for allergen-specific Immunoglobulin E (sIgE), with four allergen mixes being employed (dust mite, grass, mold, and animal epithelium). Atopy status was defined as positive if a serum sIgE value of 0.35 KU/L or more was detected for any of the antigen mixes. Asthma status was defined as positive if the patient currently used inhaled asthma medications or had a known FEV1 increase >15% after bronchodilator challenge. Smoking status was determined if the patient currently used tobacco in any form, or if they had had ceased regular tobacco use within the previous twelve months. Aspirin sensitivity was defined as positive if the patient recalled respiratory exacerbation to aspirin or other non-steroidal anti-inflammatory drugs or had a positive lysine-aspirin challenge test.
MRI assessment
Three-month follow-up T2-weighted MRI scans were used for MRI assessment. Each post-surgical cavity was considered to consist of up to 6 walls (left, right, superior, inferior, anterior and posterior), and the thickness of the mucosa was measured perpendicularly from the orientation of the corresponding wall to the point of maximal mucosal thickness (Figure 1). Nasoseptal flaps were used to reconstruct the skull base in some cases and were included in assessment of mucosal thickness. The nasal floor and septum from which these flaps were harvested was not considered to be part of the surgical cavity. Measurements were made using the digital ‘caliper’ function integrated within the DICOM reader software, InteleViewer (InteleRad, Canada), and were recorded in millimeters to one decimal point. MRI interpretation was performed blinded to endoscopic assessment and patient reported outcome measures.
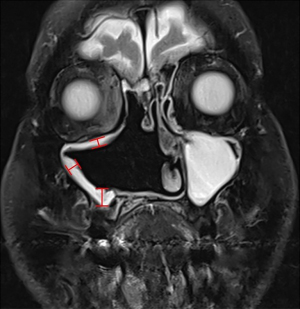
Endoscopic evaluation
Retrospective review of endoscopic video, blinded to MRI and clinical outcome measures, was used to score each wall of the post-surgical cavity via the Modified Lund-Mackay Endoscopic Score (MLMES) as defined by Snidvongs et al., a validated score sensitive to change and the patient experience (10). The MLMES comprises three subdomains—mucosa [0–6], discharge [0–2], and purulence [0 or 2]. The mucosa subdomain assesses mucosal inflammation and is composed of a seven-point ordinal scale, ranging from normal mucosa [0] to moderate oedema [3] to polypoid oedematous changes [4] to polyps extending beyond the cavity [6]. The Discharge subdomain assesses whether there is no discharge present [0], clear, thin discharge present [1], or thick, purulent discharge present [2]. The Purulence subdomain is a dichotomous score assessing whether purulent discharge is either not present [0] or present [2]. The MLMES score for each wall of the post-surgical cavity comprised the sum of the three subdomains, giving a total possible score of 10.
Patient reported outcome measures
Patient reported symptoms were measured using the Sino-Nasal Outcome Test-22 (SNOT-22), which produces a score ranging from 0 to 110 (11). Pre-operative symptoms were not assessed as they could be confounded by tumor-related sinonasal dysfunction. Subdomains within the SNOT-22 were also analyzed, including the rhinitis, ear and facial pain, sleep, psychological and nasal symptoms subdomains (12,13). All questions within the SNOT-22 were assessed on a 6-point ordinal scale, ranging from 0 (‘no problem’) to 5 (‘problem as bad as it can be’).
Statistical analysis
Statistical analysis was conducted using SPSS 27 (SPSS Inc., Chicago). Continuous parametric data were expressed as mean ± standard deviation, non-parametric continuous data were expressed as median [interquartile range (IQR)], categorical data were expressed as percentages and ordinal data were expressed as medians. As MRI mucosal thickness, MLMES and SNOT-22 were not normally distributed as per the Shapiro-Wilk test (P<0.05), correlations between continuous variables were calculated using Spearman’s correlation coefficient. The clinical utility of MRI mucosal thickness to detect clinically significant inflammation was assessed using a receiver operational characteristic (ROC) curve. Clinically significant inflammation was defined using a threshold of ≥3 of the Mucosal MLMES subdomains on the corresponding wall. As such, the endoscopic appearance needed to be normal or near normal otherwise it was defined as inflammatory changes. The maximal Youden index (sensitivity + specificity – 1) was used to select the optimal diagnostic threshold and diagnostic performance measures including sensitivity and specificity were calculated (14).
Potential confounding factors, such as atopy status, were assessed by testing for differences in outcome variables between groups. Differences in MRI mucosal thickness, total MLMES, total SNOT-22 and subdomains of the SNOT-22 were assessed using the Mann-Whitney U test. Differences between the population groups in the ordinal subdomains of the MLMES were tested via the Kendall’s Tau-b test, while the nominal subdomain of purulence was tested using Fisher’s Exact test.
Results
Seventy-two patients were assessed (58.1±17.8 years, 50% female). Asthma was present in 3 (4%) patients, smoking in 7 (10%) patients and atopy in 24 (33%). Eight (11%) patients received adjuvant radiotherapy. The median maximal MRI mucosal thickness was 2.6 (IQR 1.9) mm.
Relationship between MRI and endoscopic evidence of inflammation
A weak positive correlation was detected between MRI mucosal thickness and endoscopic assessment of the corresponding wall (rs=0.182, P<0.001). MRI mucosal thickness demonstrated weak positive correlations with the MLMES subdomains of mucosa (rs=0.207, P<0.001) and purulence (rs=0.155, P=0.005) but not discharge (rs=0.051, P=0.362).
Relationship between MRI and patient reported outcome measures
MRI mucosal thickness was not found to correlate with patient reported outcome measures assessed via the SNOT-22 (rs=−0.040, P=0.450). A very weak negative correlation was detected between MRI mucosal thickness and the SNOT-22 subdomain of sleep symptoms (rs=−0.124, P=0.019), but no correlation was detected between MRI assessment and the other SNOT-22 subdomains of rhinitis symptoms (rs=−0.010, P=0.850), ear/facial symptoms (rs=−0.016, P=0.765), psychological symptoms (rs=−0.061, P=0.249), and nasal symptoms (rs=0.066, P=0.216). No individual wall, of the surgical cavity, assessed via MRI was found to correlate with the SNOT-22: left (rs=−0.021, P=0.860), right (rs=−0.180, P=0.133), inferior (rs=0.140, P=0.240), superior (rs=−0.212, P=0.661), and posterior (rs=0.055, P=0.646).
Diagnostic utility of MRI for inflammation
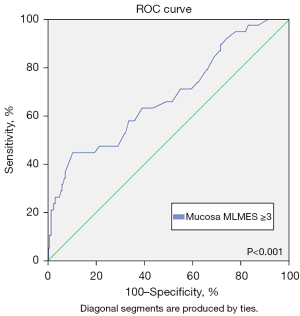
ROC curve analysis indicated that MRI mucosal thickness was of low to moderate utility for detecting clinically significant inflammation, Mucosa MLMES ≥3, with an area under the curve (AUC) of 0.678 [95% confidence interval (CI): 0.581–0.776, P<0.001] (Figure 2). As assessed by the Youden index, the optimal diagnostic threshold for clinically significant inflammation was MRI mucosal thickness ≥4 mm. This threshold demonstrated 45% sensitivity, 83% specificity, 24% positive predictive value, 77% negative predictive value, 2.69 positive likelihood ratio, 0.66 negative likelihood ratio and 4.08 diagnostic odds ratio.
Atopic vs. non-atopic status
Comparison of atopic and non-atopic patients revealed similar mucosal thickness (median 2.5 vs. 2.7 mm, P=0.952), endoscopic assessment by MLMES (median 1.0 vs. 1.0, P=0.474) and total SNOT-22 (median 21.5 vs. 17.0, P=0.361). In addition, atopic and non-atopic patients demonstrated similar sub domains of the SNOT-22: rhinitis (median 2.0 vs. 2.5, P=0.426), sleep (median 4.0 vs. 3.0, P=0.933), ear and facial pain (median 4.0 vs. 3.0, P=0.658), psychological (median 6.0 vs. 5.0, P=0.901), and nasal symptoms (median 3.0 vs. 4.0, P=0.923).
Discussion
This study aimed to determine the clinical significance of MRI thickening of the sinus cavity mucosa by using endoscopic examination and patient reported symptoms in a population with no CRS. Although MRI mucosal thickness was weakly correlated with endoscopic assessment, minor mucosal thickening (<4 mm) did not represent inflammation in most cases (77% negative predictive value). In addition, MRI mucosal thickening was not correlated with SNOT-22 symptom scoring. These findings suggest that although MRI sinonasal mucosal thickening is common, radiological thickening (<4 mm) alone does not indicate “sinusitis” in many cases.
Previous studies have emphasized that incidentally detected mucosal thickening in asymptomatic patients is not clinically significant. Various definitions have been employed to define clinically significant inflammation including thickness thresholds between ≥2 and ≥5 mm (15-19), and thresholds between ≥1/3 and ≥2/3 of the cross-sectional area of the sinus (6-8,20). However, previous studies have relied upon patient reported symptoms or limited anterior rhinoscopy to diagnose inflammation, without endoscopic confirmation (6-8,21). The single previous study that employed validated endoscopic examination included only cystic fibrosis patients with chronic sinonasal dysfunction (22). In order to endoscopically confirm that incidentally detected MRI mucosal thickening does not represent clinically significant inflammation, a population with endoscopically accessible sinuses but no chronic inflammatory sinus disease is required. This study included post-operative tumour patients, who have had their sinus cavities opened for resection of their disease but without CRS, to provide the first endoscopic assessment of the clinical significance of MRI mucosal thickening.
The lack of correlation between MRI assessment and patient symptoms noted in this study is consistent with the literature. Some previous studies have identified a lack of correlation between SNOT-22 and objective measures, such as endoscopy and CT assessment (23-25). Several articles have found that postoperative endoscopy, as assessed by the Lund Kennedy score, the Modified Lund Kennedy Endoscopic Score, or the MLMES, is better correlated with SNOT-22 and particularly the Rhinologic and Extranasal subdomains (10,25,26). However, these studies only use CRS patients, while this current study excluded CRS patients to assess changes only in those in whom no underlying condition might contribute to symptom adaptation. In conjunction with these previous findings, this study affirms that radiology alone is not sufficient for a determination of sinusitis and should be used alongside clinical evaluation to determine those in need of investigation and management.
This study is associated with certain limitations. Measurement of MRI outcomes was conducted once by a single investigator, meaning that the inter-rater and intra-rater reliability of MRI measurement could not be determined. In addition, given that most patients in this study underwent central skull base or ethmoid tumor resections, relatively few frontal sinuses were included in analysis. Surgical cavity walls that were reconstructed using nasoseptal flaps were not excluded from analysis, introducing a potential confounder that must be considered.
Conclusions
MRI mucosal thickening is weakly correlated to inflammation on endoscopic evaluation and not correlated with sinonasal symptoms. Incidentally detected minor mucosal thickening <4 mm does not indicate inflammation in most cases. Radiological findings should be used alongside clinical evaluation to make a determination of sinusitis.
Acknowledgments
The authors would like to thank both the staff of the Sydney Ear Nose & Throat clinic, and members of the Rhinology group at the St Vincent’s Centre for Applied Medical Research for their support.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-22-9/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-22-9/dss
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE’s unified disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-22-9/coif). RH serves as consultant/advisory board with Medtronic, Novartis and Meda pharmaceuticals and as the Editor-in-Chief of Australian Journal of Otolaryngology. He receives research grant funding from Glaxo-Smith-Kline and has been on the speakers’ bureau for Glaxo-Smith-Kline, Astra-zeneca, Meda Pharmaceuticals and Seqirus. LK is on the speakers’ bureau for Care Pharmaceuticals, Mylan and Seqirus Pharmaceuticals, and he serves as an unpaid editorial board member of Australian Journal of Otolaryngology from January 2019 to December 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the declaration of Helsinki (as revised in 2013). The study was approved by the St Vincent’s Hospital Human Research Ethics Committee (SVH HREC 09/083). All participants provided informed and written consent for their use of their medical data for research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim SH, Oh JS, Jang YJ. Incidence and Radiological Findings of Incidental Sinus Opacifications in Patients Undergoing Septoplasty or Septorhinoplasty. Ann Otol Rhinol Laryngol 2020;129:122-7. [Crossref] [PubMed]
- Ritter A, Rozendorn N, Avishai G, et al. Preoperative Maxillary Sinus Imaging and the Outcome of Sinus Floor Augmentation and Dental Implants in Asymptomatic Patients. Ann Otol Rhinol Laryngol 2020;129:209-15. [Crossref] [PubMed]
- Razi B, Perkovic A, Alvarado R, et al. Sinus Radiological Findings in General Asymptomatic Populations: A Systematic Review of Incidental Mucosal Changes. Otolaryngol Head Neck Surg 2022;167:16-24. [Crossref] [PubMed]
- Hansen AG, Helvik AS, Nordgård S, et al. Incidental findings in MRI of the paranasal sinuses in adults: a population-based study (HUNT MRI). BMC Ear Nose Throat Disord 2014;14:13. [Crossref] [PubMed]
- McNeill E, O'Hara J, Carrie S. The significance of MRI findings for non-rhinological disease. Clin Otolaryngol 2006;31:292-6; discussion 296. [Crossref] [PubMed]
- Wani MK, Ruckenstein MJ, Parikh S. Magnetic resonance imaging of the paranasal sinuses: incidental abnormalities and their relationship to patient symptoms. J Otolaryngol 2001;30:257-62. [Crossref] [PubMed]
- Manning SC, Biavati MJ, Phillips DL. Correlation of clinical sinusitis signs and symptoms to imaging findings in pediatric patients. Int J Pediatr Otorhinolaryngol 1996;37:65-74. [Crossref] [PubMed]
- Cooke LD, Hadley DM. MRI of the paranasal sinuses: incidental abnormalities and their relationship to symptoms. J Laryngol Otol 1991;105:278-81. [Crossref] [PubMed]
- Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012;50:1-12. [Crossref] [PubMed]
- Snidvongs K, Dalgorf D, Kalish L, et al. Modified Lund Mackay Postoperative Endoscopy Score for defining inflammatory burden in chronic rhinosinusitis. Rhinology 2014;52:53-9. [Crossref] [PubMed]
- Hopkins C, Browne JP, Slack R, et al. The national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Clin Otolaryngol 2006;31:390-8. [Crossref] [PubMed]
- Baguley C, Brownlow A, Yeung K, et al. The fate of chronic rhinosinusitis sufferers after maximal medical therapy. Int Forum Allergy Rhinol 2014;4:525-32. [Crossref] [PubMed]
- Barham HP, Osborn JL, Snidvongs K, et al. Remodeling changes of the upper airway with chronic rhinosinusitis. Int Forum Allergy Rhinol 2015;5:565-72. [Crossref] [PubMed]
- Ruopp MD, Perkins NJ, Whitcomb BW, et al. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J 2008;50:419-30. [Crossref] [PubMed]
- Tarp B, Fiirgaard B, Christensen T, et al. The prevalence and significance of incidental paranasal sinus abnormalities on MRI. Rhinology 2000;38:33-8. [PubMed]
- Patel K, Chavda SV, Violaris N, et al. Incidental paranasal sinus inflammatory changes in a British population. J Laryngol Otol 1996;110:649-51. [Crossref] [PubMed]
- Gordts F, Clement PA, Destryker A, et al. Prevalence of sinusitis signs on MRI in a non-ENT paediatric population. Rhinology 1997;35:154-7. [PubMed]
- Gordts F, Clement PA, Buisseret T. Prevalence of paranasal sinus abnormalities on MRI in a non-ENT population. Acta Otorhinolaryngol Belg 1996;50:167-70. [PubMed]
- Rak KM, Newell JD 2nd, Yakes WF, et al. Paranasal sinuses on MR images of the brain: significance of mucosal thickening. AJR Am J Roentgenol 1991;156:381-4. [Crossref] [PubMed]
- Kristo A, Uhari M, Luotonen J, et al. Paranasal sinus findings in children during respiratory infection evaluated with magnetic resonance imaging. Pediatrics 2003;111:e586-9. [Crossref] [PubMed]
- Nazri M, Bux SI, Tengku-Kamalden TF, et al. Incidental detection of sinus mucosal abnormalities on CT and MRI imaging of the head. Quant Imaging Med Surg 2013;3:82-8. [PubMed]
- Casserly P, Harrison M, O'Connell O, et al. Nasal endoscopy and paranasal sinus computerised tomography (CT) findings in an Irish cystic fibrosis adult patient group. Eur Arch Otorhinolaryngol 2015;272:3353-9. [Crossref] [PubMed]
- Psaltis AJ, Li G, Vaezeafshar R, et al. Modification of the Lund-Kennedy endoscopic scoring system improves its reliability and correlation with patient-reported outcome measures. Laryngoscope 2014;124:2216-23. [Crossref] [PubMed]
- Ryan WR, Ramachandra T, Hwang PH. Correlations between symptoms, nasal endoscopy, and in-office computed tomography in post-surgical chronic rhinosinusitis patients. Laryngoscope 2011;121:674-8. [Crossref] [PubMed]
- Schlosser RJ, Storck K, Smith TL, et al. Impact of postoperative endoscopy upon clinical outcomes after endoscopic sinus surgery. Int Forum Allergy Rhinol 2016;6:115-23. [Crossref] [PubMed]
- Toros SZ, Bölükbasi S, Naiboğlu B, et al. Comparative outcomes of endoscopic sinus surgery in patients with chronic sinusitis and nasal polyps. Eur Arch Otorhinolaryngol 2007;264:1003-8. [Crossref] [PubMed]
Cite this article as: Chang N, Matchett I, Alvarado R, Mangussi-Gomes J, Ahmadi N, Oakley G, Kalish L, Harvey R. Sinus mucosal thickening on magnetic resonance imaging and inflammation. Aust J Otolaryngol 2022;5:32.


