
Clinical application of pharyngeal high-resolution manometry in Ear, Nose and Throat (ENT) practice
Introduction
The diagnosis and management of oropharyngeal swallowing difficulty (dysphagia) is challenging (1). This is because dysphagia is not a distinct disease but rather a symptom associated with a wide range of underlying pathologies across the lifespan presenting with differing severity and permanence (1). Dysphagia has a reported prevalence of 16−20% in the general population but increases to as high as 50% in some specific groups (2), and is associated with malnutrition, dehydration and aspiration pneumonia (3,4).
A patient with dysphagia may present to an Otolaryngologist for assessment and treatment. Following exclusion of malignancy or anatomical abnormalities, swallowing studies are conducted to visualise bolus transit through the oropharynx to account for the reported dysphagia. A videofluoroscopic swallowing study (VFSS) or a flexible endoscopic evaluation of swallowing (FEES) are widely used instrumental assessments, but they provide limited insight into the biomechanical breakdown (5). Despite advancements in quantitative reporting tools (6,7) for visual instrumental assessments, there remains no globally accepted measures, leaving clinical interpretation of the swallow beyond identifying penetration and aspiration, predominantly dependent on clinician experience (8,9).
Pharyngeal high-resolution manometry with impedance (P-HRM-I) provides precise and quantitative measures of pharyngeal and upper oesophageal sphincter (UOS) pressures integrated with bolus transit (10). It can identify and localise alterations in the swallowing mechanism and determine the underlying pathophysiological breakdown leading to dysphagia. P-HRM-I metrics have been demonstrated to correlate with and be a predictor of aspiration on concurrent videofluoroscopic studies (10-13). This emerging technology operates as part of routine clinical care in only a few centers internationally (8,9,14) and requires continued clinical uptake to support translational outcomes. This paper aims to showcase the interpretation of P-HRM-I through the presentation of selected cases relevant to the Ear, Nose and Throat (ENT) practice. Specifically, new insights offered from the P-HRM-I assessment for pharyngeal dysphagia patient management beyond VFSS are discussed.
Methods
P-HRM-I
P-HRM-I has demonstrated the capability to identify altered biomechanical features contributing to dysphagia in patient cohorts (15,16) and, more recently, as an interventional outcome measure (17-20). The increased recognition of high-resolution manometry (HRM) technology for pharyngeal dysphagia is foreseeable considering HRM is considered the gold standard for the diagnosis of oesophageal motility disorders in the clinical and research settings (21).
P-HRM-I is trans-nasal catheter assessment of pharyngeal swallowing. P-HRM-I involves data acquisition from closely spaced pressure sensors to measure the contractile activity representative of pressure generation spanning the pharynx to the UOS (22). P-HRM-I simultaneously measures the pharyngeal and UOS contractile muscle activity (pressure generation) with intra-luminal impedance that is representative of bolus movement across time (10,23). Together with analysis algorithms, these pharyngeal swallowing measurements allow for a sophisticated and quantifiable biomechanical assessment that ultimately increases the understanding of swallowing physiology and pathophysiology (24,25).
Case examples have been extracted from studies conducted at two centres: Flinders Medical Centre (Adelaide, Australia) and St. George Hospital (Sydney, Australia) (approved by Southern Adelaide Clinical Human Research Ethics Committee and St. Vincent’s Hospital Human Research Ethics Committee). Informed consents are not required due to the retrospective nature of this study.
Equipment and procedure
A P-HRM-I swallow assessment can be conducted at the bedside or in the outpatient clinic setting with the patient sitting upright. P-HRM-I has been demonstrated to have high patient tolerability, with similarly low rates of complications (gagging 14%, vomiting 2% and epistaxis <1%) (22) as those reported for other trans-nasal procedures, such as nasoendoscopy. In our experience, complication rates are far lower and only occur during placement of the catheter and will resolve once the catheter is appropriately placed.
At our centre, a P-HRM-I assessment is standardised, consistent with the recommendations from the High-Resolution Pharyngeal Manometry International Working Group (24). An 8-French catheter with 32 pressure sensors (unidirectional) and 16 impedance transducers (Unisensor AG catheter, Atticon, Switzerland) is used for recording pressure and impedance data. Pressure and impedance data are acquired at 20 samples per second using the Solar GI acquisition unit (Medical Measurement System, Enschede, The Netherlands). Following a four-hour fasting period, lignocaine (5%; 2–3 sprays, equivalent to 0.3 mL) is topically administered to the nasal passage to aid catheter intubation and maximize patient comfort (24). Prior to trans-nasal catheter insertion, the catheter is placed in 37 °C water bath to reduce measurement error due to thermal drift. To assist placement of the P-HRM-I catheter, the patient is asked to place their head in a chin-down position once the catheter is advanced to velum (approximately 15 cm) to advance along the pharynx. At this time, the patient is asked to swallow sips of water as the catheter transits through the UOS into the proximal oesophagus (10,24). A minimum 5-minute accommodation period allows for subsidence of the anaesthetic effects from the lidocaine administration and participant accommodation to the catheter.
The swallowing assessment involves the administration of a standard bolus medium (SBMkitTM, Trisco Foods Pty Ltd., Brisbane, Australia) of an apple-flavored saline solution (0.65% sodium chloride NaCl) providing stable conductivity across bolus viscosity levels. Thin liquids and extremely thick liquids are prepared using Precise Thick-N INSTANT from the SBMkitTM and consistent with the International Dysphagia Diet Standardization Initiative (IDDSI) definitions (26). The standardised protocol includes testing a total of 18 boluses of 5-, 10- and 20-mL volumes of thin (IDDSI 0) and extremely thick (IDDSI 4) liquids. Three repeat swallows of each liquid volume and viscosity are recommended for acquisition of valid data (24). The duration of the procedure is approximately 15–20 minutes and can be performed in an ambulatory clinic setting.
Analysis
Currently, there are two international groups, one in Madison, USA (27,28) and the other in Adelaide, Australia (10) that use automatic methods of data extraction and analysis software platforms via MATLAB® (MathWorks, USA). Both groups have reported validation studies (29,30) and demonstrated good inter- and intra-rater reliability (31,32).
At our centre, we use the Swallow GatewayTM platform (http://www.swallowgateway.com/). Data are displayed as a spatiotemporal pressure-topography plot from the velopharynx to the proximal oesophagus (Figure 1). For each swallow, six spatiotemporal boundaries are defined for automated analysis. The anatomical markers include the proximal position of the velo-, meso- and hypo-pharynx and the proximal and distal margins of the UOS. Timing markers include the onset of UOS relaxation and contraction (Figure 1).
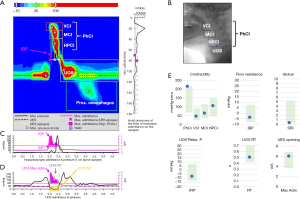
During pharyngeal swallowing, adequate pharyngeal and UOS contraction requires both longitudinal and lumen occlusive horizontal pressures (33), which can be measured using P-HRM-I (24). The lumen occlusive pressures for anatomical regions (velo-, meso-, hypo-pharynx and UOS) are measured from multiple pressure sensors (24). These pressure values are calculated as a contractile integral, which describes the ‘vigor’ of a contraction within a particular space-time box on the pressure topography plot (Figure 1). This is the mean pressure of the anatomical region multiplied by the duration (s) and length (cm) of the specified anatomical region (24). Multiple pressure sensors are also necessary for the detection of accurate UOS pressure measurements, given that the UOS is known to move superiorly between 2–2.8 cm during swallowing and that the UOS is a high-pressure zone (34).
Intraluminal electrical impedance allows for the differentiation of complete or partial bolus transit, stasis and anterograde or retrograde bolus movement (35). Intraluminal electrical impedance measures the changes in resistance (ohms), which is inversely proportional to the electrical conductivity of the luminal contents and the cross-sectional area (36). Whilst air has a high resistance to current flow (high impedance), liquids have lower resistance (low impedance). Bolus presence is measured by the lowest impedance value at the hypopharyngeal and UOS regions (10). The maximum admittance (inverse product of nadir impedance) has been reported to represent UOS opening extent during swallowing (10). Bolus presence time (BPT) is an impedance derived measure (Table 1).
Table 1
| Metric class | Metric | Unit | Definition | Clinical significance |
|---|---|---|---|---|
| Pharyngeal luminal occlusive pressures | Pharyngeal contractile integral (PhCI) | mmHg·cm·s | A measure of overall pharyngeal contractile vigor | Reduced contractile integrals highlight weakness in one or more regions of the pharynx (velo-, meso- and/or hypopharynx) or across the pharynx overall |
| Velopharyngeal contractile integral (VCI) | A measure of contractile vigor spanning the velopharynx only | |||
| Mesopharyngeal contractile integral (MCI) | A measure of contractile vigor spanning the mesopharynx only | |||
| Hypopharyngeal contractile integral (HPCI) | A measure of contractile vigor spanning the hypopharynx only | |||
| Hypopharyngeal distension pressure | Hypopharyngeal intra-bolus pressure (IBP) | mmHg | Pressure at 1 cm above to the UOS, at the time of maximum hypopharyngeal distension | Elevated values indicate increased flow resistance across the UOS |
| UOS relaxation and opening | UOS maximum admittance (UOS Max Adm) | Millisiemens (mS) | The ‘extent’ of UOS opening | Reduced values indicate reduced UOS opening extent |
| UOS integrated relaxation pressure (UOS IRP) | mmHg | A pressure measure of the extent of UOS relaxation | Elevated values indicate impaired UOS relaxation with resulting UOS restriction | |
| UOS relaxation time (UOS RT) | s | A measure of the duration of UOS relaxation | Reduced values indicate reduced duration of UOS relaxation and opening | |
| Flow timing variable* | Bolus presence time (BPT) | s | The duration that a bolus resides within the pharynx before and after swallowing (derived from impedance) | Elevated values indicate bolus presence in the hypopharynx prior to swallowing, likely due to poor oral containment, delayed pharyngeal trigger or poor pharyngeal clearance |
| Global swallowing function* | Swallow Risk Index (SRI) | – | A composite measure using to determine global swallowing dysfunction and aspiration risk | Increased values are indicative of disordered swallowing, with values over 15 indicating increased aspiration risk (on concurrent VFSS) |
*, additional metric, not part of the ‘core outcome set’. P-HRM-I, pharyngeal high-resolution manometry with impedance; UOS, upper oesophageal sphincter; VFSS, videofluoroscopic swallowing study.
The Swallow Risk Index (SRI) is indicative of overall oropharyngeal swallow function. The SRI is a validated measure, which is determined from a mathematical formula derived from the integration of pressure and impedance measures. An SRI >15 is able to identify disordered oropharyngeal swallowing and associated aspiration risk correlated with timing, weakness and obstruction aetiologies (30).
Recently, an international working group recommended a standardised testing protocol and defined set of pressure and impedance measures (‘core outcome set’) for application in both clinical and research settings, to address the variability of testing protocols (number of swallows assessed and bolus medium) and reported metrics (Table 1) (24). Since that time, normative data of asymptomatic healthy adult controls (18–79 years old) of a moderate cohort size (n=50) reported the mean and standard deviation of core and additional outcome measures (metrics not included in the core outcome set such as BPT and SRI) (37). More recently, the normative data set of a large asymptomatic cohort (n=120) reported core UOS outcome measures (25). Furthermore, access to an online analysis platform like http://www.swallowgateway.com/ allows the clinician to compare quantified results of an individual patient against these healthy ranges. It is acknowledged that ongoing normative data collection will allow the refinement of diagnostic thresholds.
Results
Case examples demonstrating P-HRM-I analysis and interpretation
These case examples demonstrate the contribution of P-HRM-I data, together with standard imaging, to guide clinical management of dysphagia commonly presenting to an ENT practice.
Case 1: a healthy pharyngeal swallow
Reconfiguration of the pharyngeal and laryngeal structures occurs during swallowing in order to propel the bolus through the pharynx and UOS, while concurrently protecting the airway (38). It is important to appreciate the manometric features present in a healthy swallow (Figure 1) in order to recognize swallowing abnormalities. The pharyngeal phase of a swallow is divided into four anatomical segments (velo-, meso-, and hypo-pharynx and UOS) (38). In Figure 1B, the VFSS image shows the P-HRM-I catheter in situ with these anatomical segments highlighted, correlating with the P-HRM-I spatiotemporal plot (Figure 1A). The spatiotemporal plot (Figure 1A) presents pressure over time with warmer colors of red and orange signifying higher pressures, and cooler colors of blue and green signify regions of lower pressures. Typically, higher pressures are generated during contractions, whereas lower pressures are observed at rest or during periods of relaxation (24). Figure 1A shows a healthy swallow manometric sequence: (I) prior to swallowing, the UOS displays tonic contractility which ceases upon initiating a swallow (represented by blue); (II) followed by a sequential pharyngeal contraction from the velo- to the hypopharynx (red); and (III) concludes with contraction of the UOS (red) which returns to the tonic baseline (green) (24).
The graphs shown below the spatiotemporal plot (Figure 1A) represent the pressure (black line) generated at the hypopharynx (Figure 1B) and at the UOS (Figure 1C). The impedance data (pink) represents bolus transit. In Figure 1B, at the hypopharynx, the bolus arrives (pink) followed by the pharyngeal contractile sequence (black). In Figure 1C, at the UOS, the bolus (pink) transits through the UOS during relaxation (black). The drop in UOS pressures to sub-atmospheric levels is represented by UOS integrated relaxation pressure (IRP). The UOS opening extent is represented by UOS maximum admittance (Max Adm) (Figure 1C) (24).
Case 2: globus sensation
Globus sensation, commonly referred to as globus, is the intermittent or persistent sensation of a lump or foreign body in the throat (39). Patients with globus commonly present to an Otolaryngologist, with prevalence reported up to 46% (40). The aetiology of globus is unclear, with contributing factors postulated to include gastro-esophageal reflux, UOS dysfunction or hypertonicity, oesophageal dysmotility, anxiety and depression (41-43).
Globus can pose a clinical challenge due to the ambiguity of self-reported symptoms, and can present with or without overt dysphagia symptoms (44). Flexible nasoendoscopy is performed to exclude a structural lesion or changes due to laryngopharyngeal reflux. In the absence of significant self-reported swallowing issues, most patients would be reassured by the Otolaryngology examination findings and discharged (41). In a small number of cases, a P-HRM-I assessment can be beneficial to exclude potential UOS dysfunction or hypertonicity contributing to globus sensation, and provides the patients with biofeedback of healthy swallowing. The P-HRM-I biofeedback is particularly helpful in those patients presenting with minor/inconsistent swallowing difficulty and globus for confirmation of a swallow within the normal range of P-HRM-I.
For a typical patient, the P-HRM-I output should demonstrate an appropriately coordinated swallow comparable to the spatio-temporal plot shown in the healthy example (Figure 1A). Furthermore, pharyngeal and UOS pressure and impedance measures should be within normal limits. An additional advantage of P-HRM-I is that the catheter can be placed to extend across the oesophagus to assess for contributory oesophageal motility disorders such as achalasia or oesophageal spasm (21).
This globus case highlights the benefit of using P-HRM-I to assess pharyngeal swallowing without the use radiation (VFSS). Additionally, P-HRM-I offers an immediate assessment of UOS dysfunction with the potential application of biofeedback for the patient (45). The clinician may review the pharyngeal topography plots of the individual swallows for features of UOS dysfunction using the recently proposed classification scheme describing UOS disorder types (25).
Case 3: cricopharyngeal bar
A cricopharyngeal bar is thickening of the cricopharyngeal muscle resulting in a narrowing of the UOS (46). Although a cricopharyngeal bar is often diagnosed as an incidental finding on fluoroscopy, in some patients it may be associated with dysphagia and aspiration (47). The reporting of a cricopharyngeal bar on imaging is commonly described as less than or greater than 50% of the lumen. In addition, a cricopharyngeal bar may be classified as non-obstructive and obstructive (48). This is dependent on the degree of narrowing at the cricopharyngeal region which may impede bolus transit, with subsequent retrograde bolus movement misdirected towards the larynx (49) potentially resulting in residue and aspiration. Surgical options include myotomy and dilatation of the UOS (50,51). However, identifying those patients who are most likely to benefit is unclear (47).
Case 3a presents a patient with self-reported worsening dysphagia and on imaging a “small” (<50% of the lumen) cricopharyngeal bar was observed (Figure 2). A cricopharyngeal dilatation did not improve symptoms, questioning subsequent treatment planning. A P-HRM-I swallowing study, in this instance, may provide additional data to ascertain the contribution of the cricopharyngeal bar to swallowing. Biomechanical pressure, timing and impedance metrics measured at the UOS demonstrate values within the normal ranges, inferring adequate UOS function. Therefore, the cricopharyngeal bar at the level of the UOS is not compromising bolus transit through the UOS, and not contributing to dysphagia for this patient (non-obstructive cricopharyngeal bar). Similarly, pharyngeal contractile pressures are within the normal ranges. However, BPT, the duration of the bolus in the pharynx prior to commencing the pharyngeal swallow sequence, is prolonged. This suggests that impaired lingual bolus control is the underlying contributing mechanism for dysphagia. In this case, surgical intervention at the UOS may not be indicated and alternative management of swallowing exercises and dietary modifications could be more appropriate.
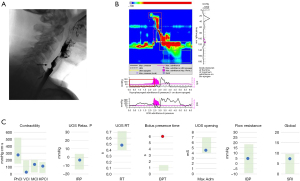
In comparison, Case 3b highlights a patient with a “significant/large” (>50% of the lumen) cricopharyngeal bar shown on imaging (Figure 3). Biomechanical pressures, timing and impedance metrics at the UOS demonstrated abnormal values, implying UOS dysfunction. The cricopharyngeal bar, in this case, is associated with impaired bolus transit through the UOS, evidenced by elevated UOS IRP and hypopharyngeal intrabolus pressures (IBP), contributing to the abnormal global SRI (obstructive cricopharyngeal bar). Furthermore, the spatiotemporal plot shows a sustained pharyngeal contraction with elevated pressures (black line) shown at the hypopharyngeal and UOS graphs. In this case, the P-HRM-I findings concur with imaging that surgical intervention at the UOS is indicated.
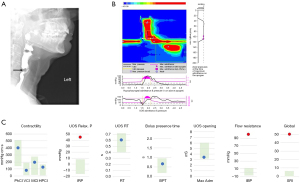
These cases highlight the added value of a P-HRM-I assessment in patients with a cricopharyngeal bar. If a cricopharyngeal bar is contributing to dysphagia, the characteristic features are elevated UOS IRP and hypopharyngeal IBP impeding bolus transit through the UOS (52). These altered metrics have been recognized to identify those potentially suitable for surgical management (47). The imaging categorisation of a small or large cricopharyngeal bar may be an insufficient measure, however, additional P-HRM-I assessment demonstrating altered UOS measures can identify those patients who will likely benefit from surgical intervention.
Case 4: dysphagia post-head and neck cancer treatment
Dysphagia following head and neck cancer treatment is reported in up to 50% of patients (53) impacting patient’s quality of life. The presentation of dysphagia in this population varies due to the anatomical location, staging and treatment type (53). Dysphagia may manifest as a combination of tongue base dysfunction, reduced laryngeal elevation, reduced pharyngeal contraction, impaired epiglottic movement and reduced UOS opening (54,55), making management challenging. P-HRM-I can distinguish the underlying biomechanical features of dysphagia, whether localised at the pharynx or UOS to inform targeted treatment.
Case 4a illustrates impeded bolus transit (increased flow resistance) through the UOS, evidenced by elevated hypopharyngeal IBP, elevated relaxation pressure (UOS IRP) and shortened UOS relaxation duration (UOS RT) (Figure 4). However, the pharyngeal contractile pressures are within the normal ranges. In this case, these results indicate UOS dysfunction, consistent with a UOS stricture often reported in post-head and neck cancer patients (56), and the patient may benefit from dilatation (57). Notably, diagnosis of stricture on radiological imaging is inconsistent, particularly in cases where pharyngeal weakness is also present (29,58).

In contrast, Case 4b demonstrates marked reduction of the pharyngeal contractile pressures [pharyngeal contractile integral (PhCI)], specifically at the sub-anatomical regions at the velo- [velopharyngeal contractile integral (VCI)] and meso-pharynx [mesopharyngeal contractile integral (MCI)] (Figure 5). These findings are illustrative of weak pharyngeal contractile pressures, also recently reported in a post-head and neck cancer cohort (59). Impaired pressure generation at the velopharynx and mesopharynx compromises bolus propulsion through the pharynx (60,61) with resulting pharyngeal residue. The spatiotemporal plot shows a repeated swallow, indicating an attempt to clear pharyngeal residue. Similar to Case 3a, prolonged BPT represents impaired lingual bolus control. The prolonged BPT in conjunction with weak pharyngeal pressures contributes to the elevated global SRI. In this case, UOS measures were within the normal ranges. These data suggest that this patient may benefit from swallowing exercises targeting pharyngeal strength and dietary modifications. Of interest, our group utilised P-HRM-I to assess biomechanical swallowing before and after a novel tongue base augmentation procedure (62) aiming to improve pharyngeal pressure generation (63).

Discussion
P-HRM-I is gaining increasing recognition as a valuable swallowing assessment method that can provide precise and quantitative measures of swallowing biomechanics. P-HRM-I assessment extends current swallow imaging assessments by identifying the biomechanical impairment at the pharynx and/or UOS, providing considerable opportunity for clinical application.
The presented case examples illustrate P-HRM-I analysis and interpretation of patients presenting to an Otolaryngologist with dysphagia symptoms, compared to a healthy swallow. Unlike traditional imaging swallowing assessment methods, P-HRM-I discriminates pharyngeal and UOS function using pressure and impedance measures. The PhCI metrics provide direct measure of generated pressures within the pharyngeal lumen (24), in contrast to inferred dysfunction from imaging assessments. Furthermore, P-HRM-I at the UOS is considered advantageous when compared with imaging assessments (58). It is important to recognise that an abnormal P-HRM-I metric in isolation may not be clinically significant and should be considered in conjunction with patient reported symptoms and any available imaging assessments.
This paper illustrates the potential for P-HRM-I assessments in extending the interpretation of imaging swallowing assessments. These findings may facilitate the continued clinical adoption of this novel technology by Otolaryngologists. P-HRM-I can be utilised either as a stand-alone assessment or as an adjunct to fluoroscopy (VFSS). In our centre, we utilise stand-alone P-HRM-I mainly in patients with an anticipated low aspiration risk, patients who have had VFSS recently performed (10,25) or in those where VFSS is difficult to perform due to patient or location factors (intensive care unit patients). A concurrent VFSS and P-HRM-I will be conducted in patients with known severe dysphagia.
To improve translation into the clinical setting, a classification scheme has recently been proposed, categorising the biomechanical breakdown at the pharynx and UOS (25). Additionally, quantitative P-HRM-I metrics allow for longitudinal assessments of dysphagia or to evaluate and guide the true effect of an intervention (dietary modifications, targeted swallowing exercises, UOS dilatation, cricopharyngeal myotomy or Botox) on swallowing outcomes (17,18,19,20,64). These quantitative measures enable demonstration of treatment efficacy.
Despite the benefits of the P-HRM-I technology, acknowledged barriers for clinical translation include equipment cost and training (8). Training opportunities are available online to assist clinicians with analysis and interpretation. Additionally, cloud-based platforms provide for multi-disciplinary collaboration (24). It is acknowledged that P-HRM-I does not provide visualisation of the swallow. However, an advantage of P-HRM-I is that it can be performed concurrently with imaging (VFSS) (9,33,65), thereby providing a comprehensive assessment of swallowing including detection of sub-clinical changes not detected on imaging alone (18,66). We anticipate that continued and increased uptake of P-HRM-I across multiple centres may facilitate the collection of normative data, improving the generalisability of this translational swallowing assessment technology.
Conclusions
Dysphagia is often multifactorial or complex in nature. P-HRM-I extends current imaging swallowing assessments enabling the Otolaryngologist to quantify and localise the biomechanical features contributing to pharyngeal dysphagia. In the case examples shown, the analysis and interpretation of the P-HRM-I metrics discriminate impairment of pharyngeal function from UOS dysfunction, and can inform treatment decision-making for the provision of individualised management.
Acknowledgments
None.
Footnote
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-22-37/prf
Funding: This work was supported by a clinical research grant from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-22-37/coif). TO reports that the development of the swallowgateway.com website was supported by grants from the College of Medicine and Public Health, Flinders University. TO holds inventorship of the international patent family that covers the analytical methods described. The Swallow GatewayTM software service is owned and provided by Flinders University. TA serves as an unpaid Editorial Board Member of Australian Journal of Otolaryngology. TA reports that this work was supported by the clinical research grant from the Passe & Williams Foundation. EHO reports that he receives grants from Flinders Foundation Health Seeding and from Garnett Passe and Rodney Williams Memorial Foundation, and receives consulting fees and payment from Medtronic. He obtains MDPI Journal of Clinical Medicine Annual Travel Grant to attend conferences. He is also an Australian Society of Otolaryngology Head and Neck Surgeon, an exec/board member of ANZ Head and Neck Cancer Society, and a member of Laryngology Society of Australasia. The conflicts of interest he has declared above are not related to the manuscript submitted. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Case examples have been extracted from studies conducted at two centres: Flinders Medical Centre (Adelaide, Australia) and St. George Hospital (Sydney, Australia) (approved by Southern Adelaide Clinical Human Research Ethics Committee and St. Vincent’s Hospital Human Research Ethics Committee). Informed consents are not required due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roden DF, Altman KW. Causes of dysphagia among different age groups: a systematic review of the literature. Otolaryngol Clin North Am 2013;46:965-87. [Crossref] [PubMed]
- Clavé P, Shaker R. Dysphagia: current reality and scope of the problem. Nat Rev Gastroenterol Hepatol 2015;12:259-70. [Crossref] [PubMed]
- Jones E, Speyer R, Kertscher B, et al. Health-Related Quality of Life and Oropharyngeal Dysphagia: A Systematic Review. Dysphagia 2018;33:141-72. [Crossref] [PubMed]
- DiBardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care 2015;30:40-8. [Crossref] [PubMed]
- Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia 1988;2:216-9. [Crossref] [PubMed]
- Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment--MBSImp: establishing a standard. Dysphagia 2008;23:392-405. [Crossref] [PubMed]
- Leonard RJ, Kendall KA, McKenzie S, et al. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia 2000;15:146-52. [Crossref] [PubMed]
- Ciucci M, Jones CA, Malandraki GA, et al. Dysphagia Practice in 2035: Beyond Fluorography, Thickener, and Electrical Stimulation. Semin Speech Lang 2016;37:201-18. [Crossref] [PubMed]
- Knigge MA, Thibeault S, McCulloch TM. Implementation of high-resolution manometry in the clinical practice of speech language pathology. Dysphagia 2014;29:2-16. [Crossref] [PubMed]
- Cock C, Omari T. Diagnosis of Swallowing Disorders: How We Interpret Pharyngeal Manometry. Curr Gastroenterol Rep 2017;19:11. [Crossref] [PubMed]
- Omari TI, Dejaeger E, Van Beckevoort D, et al. A novel method for the nonradiological assessment of ineffective swallowing. Am J Gastroenterol 2011;106:1796-802. [Crossref] [PubMed]
- Park D, Oh Y, Ryu JS. Findings of Abnormal Videofluoroscopic Swallowing Study Identified by High-Resolution Manometry Parameters. Arch Phys Med Rehabil 2016;97:421-8. [Crossref] [PubMed]
- Lan Y, Xu G, Dou Z, et al. The correlation between manometric and videofluoroscopic measurements of the swallowing function in brainstem stroke patients with Dysphagia. J Clin Gastroenterol 2015;49:24-30. [Crossref] [PubMed]
- Jones CA, Forgues AL, Rogus-Pulia NM, et al. Correlates of Early Pharyngeal High-Resolution Manometry Adoption in Expert Speech-Language Pathologists. Dysphagia 2019;34:325-32. [Crossref] [PubMed]
- Taira K, Fujiwara K, Fukuhara T, et al. Evaluation of the pharynx and upper esophageal sphincter motility using high-resolution pharyngeal manometry for Parkinson's disease. Clin Neurol Neurosurg 2021;201:106447. [Crossref] [PubMed]
- Kunieda K, Fujishima I, Wakabayashi H, et al. Relationship Between Tongue Pressure and Pharyngeal Function Assessed Using High-Resolution Manometry in Older Dysphagia Patients with Sarcopenia: A Pilot Study. Dysphagia 2021;36:33-40. [Crossref] [PubMed]
- Wu PI, Szczesniak MM, Omari T, et al. Cricopharyngeal peroral endoscopic myotomy improves oropharyngeal dysphagia in patients with Parkinson's disease. Endosc Int Open 2021;9:E1811-9. [Crossref] [PubMed]
- Fujiwara K, Koyama S, Taira K, et al. Evaluation of pharyngeal swallowing pressure using high-resolution manometry during transoral surgery for oropharyngeal cancer. J Laryngol Otol 2021;135:153-8. [Crossref] [PubMed]
- Regan J. Impact of Sensory Stimulation on Pharyngo-esophageal Swallowing Biomechanics in Adults with Dysphagia: A High-Resolution Manometry Study. Dysphagia 2020;35:825-33. [Crossref] [PubMed]
- Matsubara K, Kumai Y, Miyamoto T, et al. The effect of a chin-down maneuver after esophagectomy on oropharyngeal swallowing pressure measured using high-resolution manometry. Auris Nasus Larynx 2020;47:141-7. [Crossref] [PubMed]
- Fox MR, Kahrilas PJ, Roman S, et al. Clinical measurement of gastrointestinal motility and function: who, when and which test? Nat Rev Gastroenterol Hepatol 2018;15:568-79. [Crossref] [PubMed]
- Knigge MA, Marvin S, Thibeault SL. Safety and Tolerability of Pharyngeal High-Resolution Manometry. Am J Speech Lang Pathol 2019;28:43-52. [Crossref] [PubMed]
- Szczesniak MM, Rommel N, Dinning PG, et al. Intraluminal impedance detects failure of pharyngeal bolus clearance during swallowing: a validation study in adults with dysphagia. Neurogastroenterol Motil 2009;21:244-52. [Crossref] [PubMed]
- Omari TI, Ciucci M, Gozdzikowska K, et al. High-Resolution Pharyngeal Manometry and Impedance: Protocols and Metrics-Recommendations of a High-Resolution Pharyngeal Manometry International Working Group. Dysphagia 2020;35:281-95. [Crossref] [PubMed]
- Omari T, Cock C, Wu P, et al. Using high resolution manometry impedance to diagnose upper esophageal sphincter and pharyngeal motor disorders. Neurogastroenterology & Motility 2023;35:e14461. [Crossref] [PubMed]
- Cichero JAY, Lam PTL, Chen J, et al. Release of updated International Dysphagia Diet Standardisation Initiative Framework (IDDSI 2.0). J Texture Stud 2020;51:195-6. [PubMed]
- McCulloch TM, Hoffman MR, Ciucci MR. High-resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann Otol Rhinol Laryngol 2010;119:369-76. [Crossref] [PubMed]
- Mielens JD, Hoffman MR, Ciucci MR, et al. Application of classification models to pharyngeal high-resolution manometry. J Speech Lang Hear Res 2012;55:892-902. [Crossref] [PubMed]
- Szczesniak MM, Wu PI, Maclean J, et al. The critical importance of pharyngeal contractile forces on the validity of intrabolus pressure as a predictor of impaired pharyngo-esophageal junction compliance. Neurogastroenterol Motil 2018;30:e13374. [Crossref] [PubMed]
- Kritas S, Dejaeger E, Tack J, et al. Objective prediction of pharyngeal swallow dysfunction in dysphagia through artificial neural network modeling. Neurogastroenterol Motil 2016;28:336-44. [Crossref] [PubMed]
- Szczesniak MM, Maclean J, Zhang T, et al. Inter-rater reliability and validity of automated impedance manometry analysis and fluoroscopy in dysphagic patients after head and neck cancer radiotherapy. Neurogastroenterol Motil 2015;27:1183-9. [Crossref] [PubMed]
- Omari TI, Savilampi J, Kokkinn K, et al. The Reliability of Pharyngeal High Resolution Manometry with Impedance for Derivation of Measures of Swallowing Function in Healthy Volunteers. Int J Otolaryngol 2016;2016:2718482. [Crossref] [PubMed]
- Kahrilas PJ, Logemann JA, Lin S, et al. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology 1992;103:128-36. [Crossref] [PubMed]
- Kahrilas PJ, Dodds WJ, Dent J, et al. Upper esophageal sphincter function during deglutition. Gastroenterology 1988;95:52-62. [Crossref] [PubMed]
- Bredenoord AJ, Hebbard GS. Technical aspects of clinical high-resolution manometry studies. Neurogastroenterol Motil 2012;24:5-10. [Crossref] [PubMed]
- Nguyen HN, Domingues GR, Lammert F. Technological insights: combined impedance manometry for esophageal motility testing-current results and further implications. World J Gastroenterol 2006;12:6266-73. [Crossref] [PubMed]
- Ferris L, Doeltgen S, Cock C, et al. Modulation of pharyngeal swallowing by bolus volume and viscosity. Am J Physiol Gastrointest Liver Physiol 2021;320:G43-53. [Crossref] [PubMed]
- Cook IJ, Kahrilas PJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology 1999;116:455-78. [Crossref] [PubMed]
- Aziz Q, Fass R, Gyawali CP, et al. Functional Esophageal Disorders. Gastroenterology 2016;S0016-5085(16)00178-5.
- Zerbib F, Rommel N, Pandolfino J, et al. ESNM/ANMS Review. Diagnosis and management of globus sensation: A clinical challenge. Neurogastroenterol Motil 2020;32:e13850. [Crossref] [PubMed]
- Rasmussen ER, Schnack DT, Ravn AT. A prospective cohort study of 122 adult patients presenting to an otolaryngologist's office with globus pharyngeus. Clin Otolaryngol 2018;43:854-60. [Crossref] [PubMed]
- Järvenpää P, Arkkila P, Aaltonen LM. Globus pharyngeus: a review of etiology, diagnostics, and treatment. Eur Arch Otorhinolaryngol 2018;275:1945-53. [Crossref] [PubMed]
- Van Daele DJ. Esophageal Manometry, pH Testing, Endoscopy, and Videofluoroscopy in Patients With Globus Sensation. Laryngoscope 2020;130:2120-5. [Crossref] [PubMed]
- Siau R, Kinshuck A, Houghton L. The assessment and management of globus pharyngeus. Br J Hosp Med (Lond) 2021;82:1-8. [Crossref] [PubMed]
- O'Rourke A, Humphries K. The use of high-resolution pharyngeal manometry as biofeedback in dysphagia therapy. Ear Nose Throat J 2017;96:56-8. [Crossref] [PubMed]
- Curtis DJ, Cruess DF, Crain M, et al. Lateral pharyngeal outpouchings: a comparison of dysphagic and asymptomatic patients. Dysphagia 1988;2:156-61. [Crossref] [PubMed]
- Allen J, Blair D, Miles A. Assessment of videofluoroscopic swallow study findings before and after cricopharyngeal myotomy. Head Neck 2017;39:1869-75. [Crossref] [PubMed]
- Belafsky PC, Rees CJ, Allen J, et al. Pharyngeal dilation in cricopharyngeus muscle dysfunction and Zenker diverticulum. Laryngoscope 2010;120:889-94. [Crossref] [PubMed]
- Allen JE. Cricopharyngeal function or dysfunction: what's the deal? Curr Opin Otolaryngol Head Neck Surg 2016;24:494-9. [Crossref] [PubMed]
- Kuhn MA, Belafsky PC. Management of cricopharyngeus muscle dysfunction. Otolaryngol Clin North Am 2013;46:1087-99. [Crossref] [PubMed]
- Berzofsky CE, Holiday RA, Pitman MJ. Variability of postoperative esophagrams after endoscopic cricopharyngeal myotomy: technique dependence. Ann Otol Rhinol Laryngol 2012;121:145-50. [Crossref] [PubMed]
- Cock C, Besanko L, Kritas S, et al. Maximum upper esophageal sphincter (UES) admittance: a non-specific marker of UES dysfunction. Neurogastroenterol Motil 2016;28:225-33. [Crossref] [PubMed]
- Baijens LWJ, Walshe M, Aaltonen LM, et al. European white paper: oropharyngeal dysphagia in head and neck cancer. Eur Arch Otorhinolaryngol 2021;278:577-616. [Crossref] [PubMed]
- Starmer HM, Tippett D, Webster K, et al. Swallowing outcomes in patients with oropharyngeal cancer undergoing organ-preservation treatment. Head Neck 2014;36:1392-7. [Crossref] [PubMed]
- Barbon CEA, Chepeha DB, Hope AJ, et al. Mechanisms of Impaired Swallowing on Thin Liquids Following Radiation Treatment for Oropharyngeal Cancer. J Speech Lang Hear Res 2020;63:2870-9. [Crossref] [PubMed]
- Nguyen NP, Smith HJ, Moltz CC, et al. Prevalence of pharyngeal and esophageal stenosis following radiation for head and neck cancer. J Otolaryngol Head Neck Surg 2008;37:219-24. [PubMed]
- Spaulding SL, Ansari E, Xing MH, et al. Diagnosis and management of pharyngoesophageal stenosis: A comprehensive approach to prophylactic, endoscopic, and reconstructive treatment options. Am J Otolaryngol 2021;42:103003. [Crossref] [PubMed]
- Szczesniak MM, Maclean J, O'Hare J, et al. Videofluoroscopic Swallow Examination Does Not Accurately Detect Cricopharyngeal Radiation Strictures. Otolaryngol Head Neck Surg 2016;155:462-5. [Crossref] [PubMed]
- Schaen-Heacock NE, Jones CA, McCulloch TM. Pharyngeal Swallowing Pressures in Patients with Radiation-Associated Dysphagia. Dysphagia 2021;36:242-9. [Crossref] [PubMed]
- May NH, Davidson KW, Pearson WG Jr, et al. Pharyngeal swallowing mechanics associated with upper esophageal sphincter pressure wave. Head Neck 2020;42:467-75. [Crossref] [PubMed]
- McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope 1988;98:71-8. [Crossref] [PubMed]
- Schar MS, Omari TI, Woods CM, et al. Pharyngeal tongue base augmentation for dysphagia therapy: A prospective case series in patients post head and neck cancer treatment. Head Neck 2022;44:1871-84. [Crossref] [PubMed]
- Kraaijenga SA, Lapid O, van der Molen L, et al. Feasibility and potential value of lipofilling in post-treatment oropharyngeal dysfunction. Laryngoscope 2016;126:2672-8. [Crossref] [PubMed]
- Geng Z, Hoffman MR, Jones CA, et al. Three-dimensional analysis of pharyngeal high-resolution manometry data. Laryngoscope 2013;123:1746-53. [Crossref] [PubMed]
- McConnel FM, Cerenko D, Jackson RT, et al. Clinical application of the manofluorogram. Laryngoscope 1988;98:705-11. [Crossref] [PubMed]
- Romain D, Evans LK, Diaz Y, et al. Biofeedback Training Improves Swallowing in a Unique Case of Upper Esophageal Sphincter Hypotonicity. Laryngoscope 2021;131:E1567-9. [Crossref] [PubMed]
Cite this article as: Cheriyan SS, Schar M, Woods CM, Szczesniak M, Cock C, Omari TI, Athanasiadis T, Ooi EH. Clinical application of pharyngeal high-resolution manometry in Ear, Nose and Throat (ENT) practice. Aust J Otolaryngol 2023;6:4.


