Pinna perichondritis in a rural setting: a 10-year review of cases and an introduction to the PinCH as a novel technique for improving cosmetic outcomes following pinna abscess
Introduction
Perichondritis of the pinna is a complication of blunt or piercing trauma which can quickly lead to abscess formation. When pus collects it elevates the perichondrium off the underlying avascular cartilage, leading to direct consumption of cartilage by infection, or cartilage ischaemia due to lack of oxygenation and eventual necrosis (1). This is often associated with a long-term cosmetic deformity known as cauliflower ear, where “neocartilage” in the form of fibrosis develops beneath the perichondrium and results in unsightly thickening of the pinna with loss of normal contours (2). Complications at the site of cartilaginous pinna piercing occur in up to 35% of cases (1).
Cartilaginous ear piercing has grown in popularity in recent decades (3), and an increase in associated complications has been seen worldwide (4). Piercing of the pinna cartilage has a higher complication rate than lobe piercing (5) and is associated with Pseudomonas aeruginosa (P.aeruginosa) infection while lobule infection is commonly due to Staphylococcus aureus, including methicillin-resistant S.aureus (MRSA) (1). Despite this, many patients presenting with piercing associated perichondritis are prescribed first line antibiotics that do not adequately cover P.aeruginosa (5-7), potentially contributing to progressive infection and preventable cosmetic deformity. Industrial piercing is a modern trend where a single straight bar is passed through two separate regions of the helix.
While traditionally performed for cultural or religious reasons (8), the majority of contemporary Western cartilaginous ear piercing is done for fashion purposes (9), commonly by young females (10). Poor cosmetic outcomes from infective complications are understandably not tolerated in this patient population (11). Reconstruction of cartilage defects following abscess is a complex surgical procedure (12), requiring costal or conchal cartilage grafts (11) and often only possible in the absence of cauliflower ear (10). Despite surgical correction, a degree of deformity may still be present. Traditional methods of providing compression to pinna abscesses post-drainage to preserve the natural contours include foam or gauze bolsters secured to the pinna using quilting sutures, or worn beneath head bandages, and often require multiple follow-up visits for dressing changes as they are challenging for patients to manage themselves. Patients may find these dressings unsightly and uncomfortable. Management of piercing-associated pinna abscesses should therefore aim to both treat the infection and preserve the appearance of the pinna, as well as be available in resource-poor areas such as rural and remote Australia. To date, no methods of pinna abscess management described in the literature have addressed these aspects in a way that is easily facilitated outside of a major metropolitan centre.
The primary aim of this paper is to present 10-year retrospective review of perichondritis and pinna abscess experience in a large rural setting in order to identify risk factors for this condition and its complications, common organisms, antibiotic use and management strategies. The secondary aim is to describe a novel technique used in two of our patients for the application of compression following abscess drainage to preserve pinna contour: a moulded finger-cot splint herein described as the pinna cot-splint hack (PinCH). A review of the available literature with a focus on piercing-associated infections is presented. We present this article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-22-41/rc).
Methods
A retrospective analysis of perichondritis cases presenting to Dubbo Base Hospital Emergency Department was undertaken using the Cerner Discern Analytics 2.0 program, integrated into Cerner PowerChart electronic medical record (EMR). Patient presentations between January 2012 and January 2022 were searched first using the Emergency diagnosis term “perichondritis” then subsequently with the additional diagnosis terms “abscess of external ear”, “abscess of pinna”, “acute infection of pinna”, “acute perichondritis of pinna”, “perichondritis of external ear”, “cellulitis of pinna of ear”, “chronic infection of pinna”, “disorder of pinna” “complication of pierced pinna” and “infection of pierced pinna”. Encounters were reviewed on EMR, and considered eligible for inclusion in the analysis if the patients’ presentations were consistent with pinna perichondritis with or without abscess. Ineligible cases such as simple otitis externa or facial cellulitis were excluded. Variables of interest were collected including age, gender, Indigenous status, cause of perichondritis, duration of symptoms, first- and second-line antibiotics, presence of abscess, whether surgical management was required, microorganism cultured and presence of deformity at the end of treatment. Surgical management was defined as formal incision and drainage of abscess with complete evacuation of infected material in operating theatres with or without general anaesthetic, or ear toileting under general anaesthetic for cases of otitis externa. Needle aspirate of abscess contents was not considered adequate surgical management and was not included in the definition. Missing data was highlighted. Data were deidentified and computed using jamovi (version 2.2.5, retrieved from https://www.jamovi.org). Statistical analyses were performed using non-parametric Mann-Whitney U-tests for the continuous variables of age and duration of symptoms (not normally distributed on Shapiro-Wilk tests), and median presented as measure of central tendency. Associations between remaining categorical variables were analysed using Pearson’s Chi-squared test or Fisher’s exact test when contingency tables contained values less than 5, using P<0.05 as a significance threshold.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was reviewed by the Executive Officer of the Greater Western Human Research Ethics Committee (GWHREC) who advised that no ethical risks requiring submission to an HREC have been identified in accordance with NSW Health Policy (GL2007_020, GWAS 2023-18). Written informed consent was obtained from the patients for the publication of this article and accompanying images.
Use of the PinCH technique
Finger cot splints are affordable and readily available in hospitals in both urban and rural settings. Following incision and drainage of a pinna abscess, we recommend that iodine-soaked ribbon gauze is packed into the cavity and removed approximately 24 hours later. A compression head bandage can be placed while awaiting microbiology results to ensure appropriate antibiotic therapy is commenced before application of the splint, but this may not be necessary. The finger cot splint is moulded to the contours of the pinna (Figure 1) and placed over the wound with an absorbent gauze dressing. The finger cot splint sits firmly on the pinna and does not require tape or bandages to hold in place. Figures 2-7 demonstrate two patients from our retrospective review cohort who had pinna abscesses, with surgical drainage and the finger cot splint used, and are further discussed in the case series below. Patients should be encouraged to wear the splint during the day for the first month and use a simple head bandage at night to prevent folding of the ear during sleep, and then advised to wear the splint as needed if any swelling is noted.
Results
Our review identified 30 cases of perichondritis of the pinna admitted to our rural hospital. Demographics are presented in Table 1, and clinical features and management are summarised in Table 2. Patients had a median age of 28.3 years (IQR 37.6 years), 60% were male and 7 (23%) patients identified as Indigenous Australians. Seven (23%) cases were associated with cartilaginous piercing and are presented in bold in Table 2. Other reported causes included otitis externa [n=6 (20%)], post-operative infection [n=3 (10%)], insect bite [n=3 (10%)], blunt trauma [n=3 (10%)], chronic suppurative otitis media [CSOM, n=1 (3%)] and relapsing polychondritis [n=1 (3%)]. In seven cases (23%) there was no clear aetiology. The median time to presentation for the 30 patients was 5 days (IQR 4 days), and this did not differ significantly between gender or Indigenous status and was not correlated with age. Of the seven Indigenous patients, four (57%) required formal incision and drainage (I&D) procedures compared to 17% of non-indigenous patients.
Table 1
| Characteristic | Value |
|---|---|
| Sex, n (%) | |
| Male | 18 (60.0) |
| Female | 12 (40.0) |
| Age (years), median (IQR) | 28.3 (37.6) |
| Aboriginal or Torres Strait Islander, n (%) | 7 (23.3) |
| Aetiology, n (%) | |
| Piercing | 7 (23.3) |
| Other | 16 (53.3) |
| Unknown | 7 (23.2) |
| Symptom duration (days), median (IQR) | 5 (4.0) |
IQR, inter-quartile range.
Table 2
| Gender, age (years) | Cause of perichondritis (piercing in bold) | ATSI | Symptom duration (days) | Community antibiotics | Micro-organism | Inpatient antibiotics | Abscess | Surgical management (where performed) | Cosmetic deformity |
|---|---|---|---|---|---|---|---|---|---|
| M, 65 | Unknown | N | – | Ciprofloxacin | – | Ceftazidime | Y | I&D + drain | Y |
| F, 71 | Otitis externa | N | 5 | Ciprofloxacin | – | Ceftazidime | N | – | N |
| M, 16 | Piercing tragus | N | 5 | Flucloxacillin | S.aureus | Ceftazidime | N | – | N |
| M, 80 | Excision of lesion | N | 2 | Ciprofloxacin | – | Ceftazidime | N | – | N |
| M, 65 | Blunt trauma | Y | 3 | Ciprofloxacin | Skin flora | Cefazolin | Y | I&D + button suture | Y |
| M, 11 | CSOM | Y | 14 | Flucloxacillin | MRSA | Ceftazidime | Y | I&D + packing | N |
| M, 12 | Insect bite | N | 3 | Augmentin DF | Skin flora | Cefazolin | N | – | N |
| F, 41 | Unknown | N | 7 | Flucloxacillin | No growth | Ertapenem | Y | I&D + simple dressing | – |
| F, 73 | Recurrent perichondritis | N | 3 | Flucloxacillin | – | Ceftazidime | N | – | N |
| F, 16 | Piercing helix | N | 14 | Flucloxacillin | P.aeruginosa | Flucloxacillin | Y | I&D + packing + pressure | n/a |
| M, 8 | Otitis externa | N | 14 | Cephalexin | S.aureus | Flucloxacillin | Y | – | N |
| M, 82 | Excision of lesion | N | 14 | Ciprofloxacin | P.aeruginosa | Tazocin | N | – | N |
| F, 21 | Piercing helix | N | 7 | Flucloxacillin | – | Flucloxacillin | N | – | n/a |
| F, 36 | Relapsing polychondritis | N | 2 | Augmentin DF | No growth | Ciprofloxacin | N | – | N |
| M, 76 | Otitis externa | N | 5 | Augmentin DF | – | Ciprofloxacin | N | – | – |
| M, 46 | Otitis externa | N | 1 | Nil | – | Ciprofloxacin | N | – | – |
| M, 50 | Recurrent perichondritis | N | 2 | Flucloxacillin | – | Ciprofloxacin | N | – | – |
| F, 53 | Insect bite | N | 3 | Flucloxacillin | MRSA | Clindamycin | N | – | – |
| M, 22 | EAC furuncle | Y | 7 | Sofradex drops | Skin flora | Augmentin DF | Y | I&D of furuncle | – |
| M, 42 | Excision of lesion | N | 3 | Nil | Gram positive cocci | Cefazolin + Augmentin DF | Y | – | N |
| M, 9 | Otitis externa | N | 7 | Ciproxin drops | P.aeruginosa + S.aureus | Ceftazidime + Ciprofloxacin | N | Ear toilet | N |
| M, 33 | Blunt trauma | N | 7 | Cephalexin | – | Cephalexin | Y | – | N |
| F, 14 | Piercing antihelix | Y | 1 | Nil | S.pyogenes | Tazocin | N | – | N |
| F, 21 | Piercing antihelix | N | 14 | Cephalexin | Herpes simplex virus | Tazocin | N | – | N |
| M, 4 | Unknown | N | 1 | Nil | – | Tazocin | N | – | N |
| F, 38 | Otitis externa | Y | 4 | Augmentin DF | – | Tazocin | N | Ear toilet | N |
| M, 16 | Insect bite | N | 7 | Cephalexin | Skin flora | Clindamycin | N | – | – |
| M, 0.5 | Abrasion | Y | 2 | Nil | MRSA | Ceftazidime + Clindamycin | Y | – | – |
| F, 14^ | Piercing industrial | Y | 7 | Augmentin DF | P.aeruginosa | Tazocin | Y | I&D + packing + splint | N |
| F, 23# | Piercing industrial | N | 35 | Flucloxacillin | P.aeruginosa | Tazocin | Y | I&D + packing + splint | N |
^, Case 1; #, Case 2. M, male; F, female; ATSI, Aboriginal or Torres Strait Islander; N, no; Y, yes; DF, Duo Forte; I&D, incision and drainage; n/a, not available.
Patients whose perichondritis was a result of cartilaginous ear piercing were more likely to be younger (median age 16.7 vs. 41.5 years, P=0.046) and female (50% vs. 5.6%, P=0.009) compared to those with other causes. Non-piercing infections were more likely to grow MRSA than Pseudomonas (30.8% vs. 15.4%) while the opposite was true for piercing infections (0% vs. 50%), though these results were not statistically significant and limited by incomplete medical records. Despite being a common cause for infection, piercing was not shown to increase the likelihood of abscess formation or need for surgical management.
Patients with pseudomonal infection were more likely to have presented later (median time 14 vs. 4 days, P=0.033) and then require surgical management (80% vs. 28.6%, P=0.046) than those without. Indigenous patients, those with abscess and those who presented later were also more likely to require formal surgery (71.4% vs. 21.7%, P=0.026; 66.7% vs. 11.1%, P=0.004; median time 7 vs. 3 days, P=0.02). Abscess formation was associated with increased time to presentation (median time 7 vs. 3.5 days, P=0.032) and was seen in 71.4% of Indigenous patients compared to 30.4% of non-indigenous patients, though this did not reach statistical significance.
Pseudomonal infection was the causative pathogen for 42.9% of infections following piercing of the pinna. Unfortunately, remarkably few patients had microbiology culture performed even when undergoing I&D for abscess. Patients receiving anti-pseudomonal antibiotics in the community were significantly older than patients who received antibiotics targeting other microorganisms (median age 71.6 vs. 21.1 years, P=0.002). Once in hospital, patients with an abscess were less likely to receive anti-pseudomonal antibiotics than those with non-suppurative perichondritis (50% vs. 83.3%, P=0.05).
Case series of two PinCH cases
Case 1
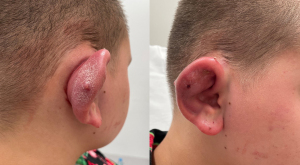
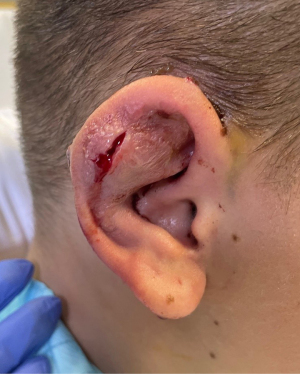
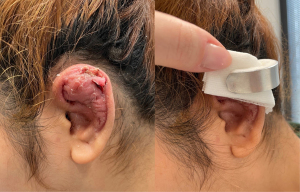
A 14-year-old female presented to our rural facility’s Emergency Department with a 5-day history of right pinna pain, erythema and increasing swelling, two weeks after an “industrial” piercing. When the symptoms began, she had removed the bar and saw her General Practitioner who prescribed flucloxacillin to no effect. She then presented to ED (Figure 2), was commenced on IV tazobactam and piperacillin (Tazocin) and underwent incision and drainage under general anaesthetic: complete loss of scaphoid and partial loss of postero-superior helical cartilage was noted. The cavity was swabbed, debrided, and irrigated with iodine and saline before iodine-soaked ribbon gauze packing was placed. This was removed after 24 hours (Figure 3), a compression head bandage placed, and the patient discharged on oral ciprofloxacin. Swabs returned positive for pure growth of P.aeruginosa sensitive to ciprofloxacin, Tazocin and ceftazidime. The patient was followed up in clinic three days post discharge and the bandage was replaced with a moulded finger cot splint, which she reported as being much more comfortable, less unsightly, and easy to apply. After one month, the patient had minimal residual swelling and by six months rated her cosmetic outcome as excellent (Figure 4).
Case 2
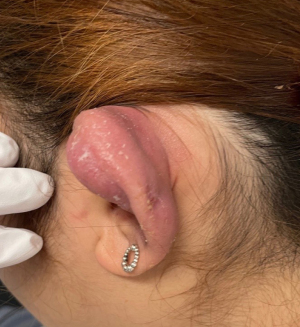
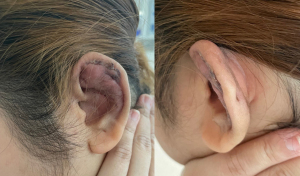
A 23-year-old female was referred to our service with a four-week history of left pinna pain swelling and erythema, after having an industrial piercing placed 6 weeks prior. She had initially presented to the Emergency Department 2 weeks after the piercing with erythema and discharge which had not responded to oral flucloxacillin, whereupon the bar was removed and the ear was cleansed with chlorhexidine. Swabs were not taken and she was continued on flucloxacillin. She represented a month later with significant swelling, erythema and pain having had no response to the second course of flucloxacillin. At this point (Figure 5) she was commenced on IV Tazocin and underwent incision and drainage. She was noted to have complete loss of scaphoid and triangular fossae cartilage, extending inferiorly to involve most of the antihelix. Necrotic debris was removed, the cavity swabbed, irrigated and packed with iodine soaked ribbon gauze and a pressure dressing was placed. Residual swelling was noted the following day, and she remained on intravenous antibiotics for a further 72 hours. The head bandage dressing was changed to the moulded finger splint (Figure 6) before she was discharged on oral ciprofloxacin once cultures returned positive for P.aeruginosa sensitive to ciprofloxacin, Tazocin and ceftazidime. At follow-up at 1 week (Figure 7) the swelling continued to improve with return of some of the normal contours of the pinna, and by 4 months there was only mild fibrosis seen at the antihelix with the patient reporting a good cosmetic outcome and better comfort using the splint compared to the compression bandage.
Discussion
Our study is the first to describe a large cohort of pinna perichondritis and abscess managed in the rural Australian setting and the increased risk for indigenous patients of perichondrial abscess. This study demonstrates the variability in presentations of pinna perichondritis and highlights that piercings are a common aetiology often associated with a pseudomonal infection.
In Table 3 we summarised the risk factors for pinna perichondritis and abscess as identified in the literature and in our study cartilage piercing was the most common cause. We have also described the novel application of the finger cot splint on two recent patients to maintain pressure on the skin and perichondrium in the post-operative period and with excellent results as seen in Figures 2-7. This technique uses easily available splints and does not require removal of stitches for the removal of bolsters. This is important as it potentially avoids a subsequent anaesthetic in children or for people with developmental delay where pinna trauma can also occur and where removal of stitches without a general anaesthetic is challenging.
Table 3
| Outcome | Risk factors |
|---|---|
| Perichondritis | Cartilaginous piercing (5) |
| Adolescent, poor patient hygiene (9) | |
| Poor hygiene by proceduralist (9) | |
| Pinna abscess | Piercing gun (13) |
| Misdiagnosis, ineffective treatment (14) | |
| Double cartilage piercing* | |
| Pseudomonas infection | Contaminated disinfectant (5) |
| Use of benzalkonium chloride (5) | |
| Upper cartilage piercing (5,15) | |
| Humidity, warmer climate (13) | |
| Cosmetic deformity | Longer time to presentation (15) |
| Abscess formation (and subsequent drainage) (16) | |
| Scaphoid piercing (17) |
*, identified in this study.
Our study highlights the variability of community and in-hospital antibiotic usage, and this is similar to the variability described in the literature. P.aeruginosa has been demonstrated to be a leading cause of cartilage piercing related infections with and without abscess (17,18), and displays antibiotic resistance to many beta-lactam antibiotics (4,19). Despite this documented prevalence, patients are commonly prescribed first-line antibiotics in the community that do not effectively target pseudomonal species (5) and this was particularly common in our study in the rural setting. Several factors may contribute to this. Clinicians may not recognise the features of perichondritis which differentiate it from cellulitis or otitis, namely sparing of the lobule, and prescribe antibiotics appropriate for the condition they believe they are treating (14). They may diagnose perichondritis but not be aware of the incidence of P.aeruginosa infection in this condition and the need to cover with anti-pseudomonal (20). They may have concerns regarding the safety of fluoroquinolones in children especially (21), and not be willing to prescribe the only oral anti-pseudomonal agents available. In many cases, this has prompted community clinicians to refer patients to hospital for intravenous antibiotics (18), giving more treatment options such as Tazocin and ceftazidime (19).
Concerns regarding the use of fluoroquinolones such as ciprofloxacin in children may be eased as the safety profile of these drugs becomes better understood (22). A 2011 systematic review of over 16,000 paediatric patients demonstrated the safe use of ciprofloxacin in this population, with only 1.6% of children experiencing a complication, 50% of which was arthralgia which resolved on cessation, and no children experiencing growth disturbance (21). According to a 2016 clinical report by the American Academy of Pediatrics, there is no evidence for long-term musculoskeletal harm from fluoroquinolones, and the rates of neurological adverse events were the same between ciprofloxacin and comparator groups (22). Ciprofloxacin has good activity against P.aeruginosa, as well as moderate activity against S.aureus (21), making it an ideal choice in post-piercing perichondritis. Appropriate prescribing of antibiotics for perichondritis is essential to reduce the development of catastrophic drug-resistant bacteria (19), and as demonstrated by the results of this study, community treatment with anti-pseudomonal antibiotics reduces the risk of simple perichondritis developing into an abscess.
Despite the ease at which an individual can obtain a pinna cartilage piercing (5,23), it should be considered a high-risk procedure for infection due to its anatomical location. Complications occur in up to 35% of piercings in a region with low blood supply (24), such as the helical rim. Infection on both sides of the cartilage with perichondrial stripping and underlying pus formation causes ischaemic necrosis of the cartilage. A series of connections between distal branches of the superficial temporal and posterior auricular arteries provide the skin and perichondrium of the helix with a watershed blood supply (25). The patients with Industrial piercings in our case series may be at a higher risk of infection than most people undergoing ear piercing, due to having two separate skin perforations made at the one time. However, if infection develops at both sites, it may cause interruption of superior and inferior blood flow from the helical arcades, leaving the middle third of the helical rim more vulnerable to ischaemia. Due to their superior position, cartilaginous piercings are also at a higher risk of contamination from hair as well as compression during sleep, both of which may promote infection (24). Our study is the first to observe that multiple simultaneous helix cartilage piercings as seen in the industrial style, may carry a higher risk of cartilage necrosis compared to single piercings.
Aside from age restrictions which vary between states and territories, clients seeking pinna piercing in Australia come across very few hurdles including no requirement for written consent or parental chaperone. Piercing technicians often do not discuss the risks of cartilaginous ear piercing with their clients, and many are not actually aware of the infective or cosmetic complications of their practices (9,26). It has been suggested that cartilaginous ear piercing be classified as an invasive procedure to improve awareness and compliance with informed consent processes (9).
Regarding the method of piercing, it has been thought that the use of a spring-loaded gun would contribute to an increased risk of perichondritis and subsequent abscess formation. The theory is that perichondrial stripping occurs more frequently with gun piercing than with a needle (6,13,23). Proceduralists at piercing and tattoo parlours, who have often undergone more training than those at jewellery kiosks, report exclusively using a needle for cartilaginous piercing for this reason (23). However, a histologic study by van Wijk et al. demonstrated no difference in the degree of cartilage shatter or perichondrial stripping (27), within the limits of cadaveric models. Both our patients with the PinCH abscess care had undergone piercing with a needle rather than a gun. Sterility appears to be better maintained at body piercing parlours using single use or autoclaved instruments and isopropyl alcohol cleansing compared to jewellery kiosks, where the antiseptic sprays used to cleanse skin and jewellery may be contaminated with pseudomonas (5,23). The higher rates of pseudomonal pinna abscess from piercings performed at jewellery kiosks may be a result of contamination rather than trauma from the actual piercing method (5,27), and prevention of complications should focus on improving hygiene practices including aftercare (9).
Poor cosmetic outcomes due to pinna abscess have previously been considered unavoidable (16,28). While quilting sutures have demonstrated good cosmetic results when used for management of pinna haematomas (2), this practice is not performed routinely for pinna abscess due to concerns regarding the trapping of residual infection and the need for suture removal requiring anaesthetic in some patients. Instead, pressure bandages are often worn once the infected material is evacuated (15), which can be uncomfortable, unsightly and—unless involving specially positioned bolsters—do not provide targeted pressure over the contours of the pinna. This is particularly important where cartilage necrosis has occurred and the helical skin may flatten with uniform pressure or collapse without external support. Patients are usually not able to place these specialised bolsters themselves and need to attend frequent follow-up visits for dressing changes, which may not be feasible in rural or remote settings. Without pressure dressing, fibrosis and thickening of the subcutaneous tissues may occur causing cauliflower ear (2), which can complicate later surgical reconstruction (10). The type of pressure dressing used must be easily accessible and well tolerated by the patient to ensure compliance and prevent this fibrosis or collapse. Cosmetic reconstruction may be able to provide acceptable cosmetic appearance in many cases of pinna abscess with cartilage loss (12), however these procedures are often costly, require costal graft harvesting (10), are only possible months after the deformity has set in (11) and rarely available in regional settings. In some cases, the deformity resulting from the infection can be so severe that surgical reconstruction is not able to achieve optimal results. A method of applying pressure which prevents fibrosis, preserves contours and reduces the need for surgical reconstruction is ideal. Aluminium finger cot splints are readily available in both urban and rural hospital settings, are low cost and easy to use. When applied using the PinCH technique, these splints provide an ideal compression method.
The PinCH technique was developed within the retrospective review study period and used for the management of piercing-associated pinna abscess in two young female patients, as detailed in the case series. For these patients, the appearance of compression head bandaging was considered unacceptable however the same concerns were not expressed towards the splint. In both cases, the splint was only applied after 72 hours of head bandaging while microbiology results were pending, over concerns that the moulded splint may trap residual infection more so than bandaging in the event of infection with resistant organisms. Thus, once culture results confirmed infection with bacterial strains susceptible to prescribed antibiotics, the PinCH technique could be used. For future cases, we will trial earlier application of the splint immediately following removal of wound packing, as well as using the method for non-infective causes of pinna collection, such as following drainage of pinna haematoma or serous fluid in traumatic or inflammatory collections. Over time with more data, we hope to quantitatively compare outcomes of the PinCH technique with conventional methods. However, having only used this method in 2 cases to date, this analysis is not currently possible.
In this retrospective study, cosmetic deformity was associated with a longer time to presentation and the presence of a collection that required surgery: both factors having been previously demonstrated in the literature (15,16). According to a 2015 systematic review, cosmetic defects of the pinna were more likely to result from piercings to the scaphoid fossa than the helical rim, suggesting that preservation of cartilage in this region is critical for cosmesis of the ear (7). Our two case series patients had multiple simultaneous piercings leading to infection with both complete loss of scaphoid fossa cartilage and some helical rim destruction, putting them at risk of significant cosmetic deformity. With the PinCH technique, we were able to prevent significant deformity and achieve good cosmetic outcomes for our patients.
The limitations of our study are small numbers, both for the larger retrospective cohort and for the two patients with the PinCH technique. The rural setting understandably does often have smaller numbers but does have the advantage of collecting all cases in the area, providing a complete collection of cases in the region. As previously stated, the PinCH technique needs further evaluation, but our preliminary use suggests it is a safe and accessible technique in settings outside major teaching hospitals. Our retrospective analysis was limited by several factors. Degree of residual deformity was often not recorded on EMR, and some patients did not have microbiology swabs taken even in the presence of abscess. Some patients were lost to follow-up or followed up outside the public hospital so records were unavailable. Various diagnostic terms were applied to patients admitted to our centre with pinna perichondritis, there remains the possibility that true perichondritis cases may have been missed due to their being misdiagnosed simply as “cellulitis”.
Conclusions
In conclusion, pinna perichondritis in the rural setting is commonly associated with cartilaginous piercing, and we have identified the industrial piercing as a risk factor potentially related to compromised blood supply between the piercings. Younger patients are routinely not prescribed anti-pseudomonal antibiotics despite high prevalence of P.aeruginosa seen in this cohort despite guidelines. Rural settings have less resources and good cosmetic outcomes are best to strive for at time of the initial treatment. We describe the PinCH technique with readily available, affordable and mouldable finger cot splints to fit to the contours of the pinna for use following formal drainage of an abscess. This method of compression may reduce the burden of cosmetic deformity and ultimately the need for surgical reconstruction, particularly in young female patients who are more likely to seek multiple cartilaginous pinna piercings and for whom cosmetic appearance of the ear has substantial importance. While it has only been demonstrated in management of piercing-associated pinna abscess thus far, the PinCH technique has potential application in other causes of pinna collection where traditional methods of compression are less feasible.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-22-41/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-22-41/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-22-41/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-22-41/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was reviewed by the Executive Officer of the Greater Western Human Research Ethics Committee (GWHREC) who advised that no ethical risks requiring submission to an HREC have been identified in accordance with NSW Health Policy (GL2007_020, GWAS 2023-18). Written informed consent was obtained from the patients for publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fernandez AP, Neto IC, Anias CR, et al. Post-piercing perichondritis. Braz J Otorhinolaryngol 2008;74:933-7. [Crossref] [PubMed]
- Roy S, Smith LP. A novel technique for treating auricular hematomas in mixed martial artists (ultimate fighters). Am J Otolaryngol 2010;31:21-4. [Crossref] [PubMed]
- Hanif J, Frosh A, Marnane C, et al. Lesson of the week: "High" ear piercing and the rising incidence of perichondritis of the pinna. BMJ 2001;322:906-7. [Crossref] [PubMed]
- Klug TE, Holm N, Greve T, et al. Perichondritis of the auricle: bacterial findings and clinical evaluation of different antibiotic regimens. Eur Arch Otorhinolaryngol 2019;276:2199-203. [Crossref] [PubMed]
- Keene WE, Markum AC, Samadpour M. Outbreak of Pseudomonas aeruginosa infections caused by commercial piercing of upper ear cartilage. JAMA 2004;291:981-5. [Crossref] [PubMed]
- Sandhu A, Gross M, Wylie J, et al. Pseudomonas aeruginosa necrotizing chondritis complicating high helical ear piercing case report: clinical and public health perspectives. Can J Public Health 2007;98:74-7. [Crossref] [PubMed]
- Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope 2015;125:1827-34. [Crossref] [PubMed]
- Chowdhury WA, Hossain MM, Chowdhury MR, et al. High ear piercing--a dangerous craze. Mymensingh Med J 2004;13:201-2. [PubMed]
- Lyons M, Stephens J, Wasson J, et al. High ear-piercing: an increasingly popular procedure with serious complications. Is good clinical practice exercised? Eur Arch Otorhinolaryngol 2012;269:1041-5. [Crossref] [PubMed]
- Lane JC, O'Toole G. Complications of ear rings. J Plast Reconstr Aesthet Surg 2012;65:747-51. [Crossref] [PubMed]
- Margulis A, Bauer BS, Alizadeh K. Ear reconstruction after auricular chondritis secondary to ear piercing. Plast Reconstr Surg 2003;111:891-7; discussion 898. [Crossref] [PubMed]
- Iida N, Hosaka Y, Ogawa T. Correction of auricular deformity caused by high ear-piercing: case report. Ann Plast Surg 2003;50:82-4. [Crossref] [PubMed]
- Rowshan HH, Keith K, Baur D, et al. Pseudomonas aeruginosa infection of the auricular cartilage caused by "high ear piercing": a case report and review of the literature. J Oral Maxillofac Surg 2008;66:543-6. [Crossref] [PubMed]
- Rivera-Morales MD, Rodríguez-Belén JL, Vera A, et al. Perichondritis: Not All Ear Pain Is Otitis. Cureus 2020;12:e11141. [Crossref] [PubMed]
- Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol 2015;272:3163-7. [Crossref] [PubMed]
- Prasad HK, Sreedharan S, Prasad HS, et al. Perichondritis of the auricle and its management. J Laryngol Otol 2007;121:530-4. [Crossref] [PubMed]
- Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol 2013;127:505-8. [Crossref] [PubMed]
- Davidi E, Paz A, Duchman H, et al. Perichondritis of the auricle: analysis of 114 cases. Isr Med Assoc J 2011;13:21-4. [PubMed]
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol 2011;12:157-69. [Crossref] [PubMed]
- Stewart GM, Thorp A, Brown L. Perichondritis--a complication of high ear piercing. Pediatr Emerg Care 2006;22:804-6. [Crossref] [PubMed]
- Adefurin A, Sammons H, Jacqz-Aigrain E, et al. Ciprofloxacin safety in paediatrics: a systematic review. Arch Dis Child 2011;96:874-80. [Crossref] [PubMed]
- Jackson MA, Schutze GE. The Use of Systemic and Topical Fluoroquinolones. Pediatrics 2016;138:e20162706. [Crossref] [PubMed]
- More DR, Seidel JS, Bryan PA. Ear-piercing techniques as a cause of auricular chondritis. Pediatr Emerg Care 1999;15:189-92. [Crossref] [PubMed]
- Stirn A. Body piercing: medical consequences and psychological motivations. Lancet 2003;361:1205-15. [Crossref] [PubMed]
- Zilinsky I, Erdmann D, Weissman O, et al. Reevaluation of the arterial blood supply of the auricle. J Anat 2017;230:315-24. [Crossref] [PubMed]
- Jervis PN, Clifton NJ, Woolford TJ. Ear deformity in children following high ear-piercing: current practice, consent issues and legislation. J Laryngol Otol 2001;115:519-21. [Crossref] [PubMed]
- van Wijk MP, Kummer JA, Kon M. Ear piercing techniques and their effect on cartilage, a histologic study. J Plast Reconstr Aesthet Surg 2008;61:S104-9. [Crossref] [PubMed]
- Perry AW, Sosin M. Reconstruction of ear deformity from post-piercing perichondritis. Arch Plast Surg 2014;41:609-12. [Crossref] [PubMed]
Cite this article as: Jaensch SL, Watson AL, Sirigiri R, Birman CS. Pinna perichondritis in a rural setting: a 10-year review of cases and an introduction to the PinCH as a novel technique for improving cosmetic outcomes following pinna abscess. Aust J Otolaryngol 2023;6:5.