
Skull base osteomyelitis in the Auckland region
Introduction
Skull base osteomyelitis (SBO) can arise from severe otitis externa, also termed “malignant otitis externa”. It is an aggressive inflammatory process which begins in the external ear in an immunocompromised patient. Otogenic SBO is characterised by spread of infection through thrombosed vessels extending from the external auditory canal (EAC) into the petrous temporal bone (1). Patients usually have severe pain which worsens despite appropriate topical treatment, and commonly present with granulation tissue in the EAC (2). Cranial nerve palsies can develop due to spread of disease to cranial nerve foramina. Diagnostic criteria vary widely. A systematic review in 2013 identified 27 publications that each employed unique criteria to make the diagnosis (3). Broadly however, the disease is characterised by a triad of clinical signs, evidence of acute inflammation measured with blood markers, and radiologic evidence of osteomyelitis.
Treatment is primarily with a prolonged intravenous (IV) antimicrobial regimen. To mitigate antimicrobial resistance; a course of treatment should comprise a high-dose, low resistance potential antimicrobial for the shortest duration (4). The rate of relapse and/or failure of SBO treatment has been up to 20% (5). We hypothesize that the cause for this in some cases is premature completion of antimicrobial therapy, and that the risk of relapse or failure may be mitigated by a longer course of targeted antimicrobial treatment. Surgery in our centre is reserved for microbial sampling to guide antibiotic choice. Some however advocate surgery for drainage of abscess, removal of sequestra (6), or to encourage neovascularisation and optimise drug delivery (7).
Historical experience of treatment outcomes has been poor, with a 46% mortality rate observed in Chandlers early series (1). Owing to improvements in antimicrobial drugs, advances in diagnostic imaging techniques, and an increasing awareness of the disease itself this had reduced to 32% by 1972 (8), and further to 15% by 2006 (9).
The markers of disease resolution, and therefore the necessary duration of antibiotic therapy is debated (10-12). Culture driven antibiotic treatment is critical in serious infections (13), however there are proponents of empiric treatment with evidence of favourable clinical outcomes in SBO (14,15).
Confirming cure after a course of treatment is challenging but is clearly crucial. Gallium or Indium nuclear scans have been utilised to assess for resolution of osteomyelitis in the skull base and are still advocated by many as a disease monitoring modality. A recent meta-analysis however found the sensitivity of Gallium 67 to predict disease resolution was only 71%, and its specificity also too low to be considered useful (16).
The aims of this study are to add to existing data on the microbiology and management of this rare disease in the Auckland region, and to identify factors that correlate with clinical success, relapse, or failure of SBO treatment. Radiologic and inflammatory outcomes are assessed in their capacity to predict clinical outcomes and thus help guide decision making on when to stop treatment. The results will serve to update the authors treatment protocol previously published (17). We present this article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-22-23/rc).
Methods
A retrospective cohort study was performed. We used the electronic medical records systems of the three public hospitals in the greater Auckland region (population 1.7 million) to identify patients who had been admitted to hospital with “Skull Base Osteomyelitis” or “Malignant Otitis Externa” between January 1, 2004 and December 31, 2019. This did include a cohort of 20 patients between 2004 and 2011 that had previously been reported on in a prior study (17). All patients with a clear clinical history, symptoms, and signs of acute otitis externa were included. These included severe otalgia, edema of the EAC, with or without the presence of granulation tissue and purulent otorrhea. To allow estimates of disease incidence we used data from the censuses performed in 2006, 2013, and 2018, plus population estimates provided by Statistics New Zealand (18) to determine the size of the Auckland regional population during each study year.
The electronic medical records of patients were retrospectively reviewed to obtain demographic (age, gender, chosen ethnicity), clinical, laboratory, imaging, treatment and outcome data for all patients. All available follow up data for a minimum of 12 months from diagnosis was gathered. Data collection commenced in early 2021 after the last recruit had completed 12 months of follow up. Regular clinical follow up of the patients occurred in both Otolaryngology and Infectious Disease clinics.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Auckland Health Research Ethics Committee (AH25092). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Clinical outcomes
Our primary outcome was clinical outcome from treatment. Patients whose medical records documented complete resolution of clinical signs of disease during treatment without subsequent relapse in the 12 months following completion were considered to have been “cured”. Those in whom disease recurred within 12 months of the completion of treatment were considered to have suffered a “relapse”, and those who continued with clinical signs of disease at the completion of treatment were deemed to have “failed”.
Radiologic outcomes
Gallium 67 bone scan fused with SPECT (single photon emission tomography) performed at, or shortly after completion of treatment reporting no abnormal uptake of gallium in the skull base were considered to represent radiographic resolution of SBO. Radiographic failure was defined as reported persistence or progression of radiographic evidence of disease on gallium scan at the conclusion of treatment.
Inflammatory outcomes
Patients were monitored with serial C-reactive protein (CRP) assays during treatment. The nadir CRP within 7 days either side of treatment completion date was used as a measure of inflammatory outcome. CRP less than 5 represented inflammatory resolution, and CRP over 5 represented inflammatory failure. This is the cut-off value used by the regional laboratory to define abnormally elevated levels. Erythrocyte sedimentation rate (ESR) was measure in only 8 patients and therefore was not analyzed.
Statistical methods
A series of chi-square tests were performed to assess for association between variables at presentation, and clinical outcome. These were: age >70 years, facial palsy, diabetes, and the presence of skull base erosion seen on CT scan, which have been reported as risk factors for severe disease (2). These are expressed as odds ratio of adverse outcome (relapse or failure). Chi-square tests were also performed to assess for associations between the radiologic outcomes, inflammatory outcomes, and the clinical outcomes observed. Smoking was also assessed for its association with clinical outcome, owing to its known adverse influence on the microvasculature. Statistical significance was defined as P value <0.05. All analyses were performed using SPSS v25 (SPSS Inc., IBM Corp., Armonk, NY, USA).
Results
We identified 63 patients who were admitted to a hospital in the Auckland region for SBO during the study period. The annual incidence of SBO increased over the study period, from 0.15 cases per 100,000 population during 2004–2008; to 0.45 cases per 100,000 population during 2014–2019. The incidence of SBO was higher in Pasifika over the same period (0.46 per 100,000 in 2004–2008 to 1.14 per 100,000 in 2014–2019) (Figure 1).

The median age at presentation was 64 years (range, 34–89 years). 76% were male, and 89% had a diagnosis of diabetes. These data are comparable to other contemporary series displayed in Table 1. Thirty-seven patients required general anesthetic to allow for biopsies to be taken for culture and histology. Eighty-one percent (30/37) were classified as American Society of Anaesthesia (ASA) grade 3 or 4, portending high perioperative risk.
Table 1
| Measure | Auckland | Stevens et al. (19) | Stern Shavit et al. (2) |
|---|---|---|---|
| Total cases | 63 | 28 | 88 |
| Male: Female | 48:15 | 24:4 | 61:27 |
| Age (years) | 64±18 | 63±19 | 73±12 |
| Age >70 | 25 (40%) | ||
| Diabetes mellitus | 56/63 (89%) | 26/28 (93%) | 66/88 (75%) |
| Granulation tissue present (EAC) | 66/88 (75%) | ||
| Smoker | 10/63 (16%) | ||
| Hba1c (mmol/mol) | 66.6 (range, 36–155) | ||
| Facial nerve palsy | 30/63 (48%) | 8/28 (29%) | 15/88 (17%) |
| CT evidence of spread to adjacent structures | 34/63 (54%) | 84% | |
| Technetium scan positive | 100% | 95% of 85 patients | |
| Pseudomonas isolated | 40/63 (63%) | 39 (44%) | |
| Fungus isolated | 26/63 (41%) | 3 (11%) | 12 (14%) |
| Treatment duration | |||
| Duration IV (median) | 42 days | 66 days | |
| Duration IV + oral (median) | 75 days | ||
| Cure rate | 44/56 (78%) | 18/28 (64%) | |
| Failure of treatment | 12/56 (21%) | 24/88 (27%)* | |
| Disease specific mortality | 7/56 (12.5%) | 5/28 (17%) | 12/88 (14%) |
| Relapse | 16/56 (28%) | 6/28 (21%) | 12/88 (14%) |
Data are expressed as n (%) or mean ± SD or mean (range). *, disease persisted beyond 6-week period. EAC, external auditory canal; CT, computed tomography; IV, intravenous antibiotic; SD, standard deviation.
Forty-nine percent (31/63) patients presented with one or more cranial nerve palsies; involving the facial (30/63=48%), vagus (4/63=6%), hypoglossal (3/63=5%), glossopharyngeal (2/63=3%), abducens (2/63=3%), and accessory nerves (1/63=2%). Fifty-four percent (34/63) patients had CT scan evidence of bone destruction/spread to adjacent structures at presentation, indicating severe disease as previously defined (19).
A total of 183 specimens were submitted to hospital microbiology laboratories prior to the commencement of inpatient IV antimicrobial therapy, a median of 3 specimens per patient (range, 1–8 specimens) (Table 2). The most frequently sampled sites were the EAC (59/63 patients =94%), mastoid (20/63=32%), postnasal space (15/63=24%), and temporomandibular joint (TMJ) (4/63=6%). Seventy-three percent (46/63) patients had one or more tissue samples submitted, while the remaining 17/63 (27%) had swabs but no tissue submitted. The relative yield of culture specimens varied by site (Table 3). Tissue samples obtained from the EAC/middle ear, and the postnasal space were positive in 94% and 73% of cases, respectively. However, positive culture rate from mastoid bone and/or mucosa was relatively low 5/19 (26%). Fifty-nine percent (37/63) had tissue biopsies obtained in the operating theatre under general anaesthesia where the rate of isolation of a pathogen i.e., positive culture was 14/37 (38%). In contrast, 18/63 (29%) had tissue biopsies obtained using local anaesthesia in a clinic or ward setting; of which 17/18 (94%) were positive.
Table 2
| Organism | Number of samples | Location of sample |
|---|---|---|
| Pseudomonas aeruginosa | 63 | EAC swab [34], canal/ME tissue [19], mastoid [6], TMJ aspirate [2], PNS tissue [2] |
| Fungi | 38 | EAC swab [27], canal tissue [6], PNS tissue [3], mastoid tissue [2] |
| Staphylococcus aureus | 11 | EAC swab [4], PNS tissue [4], EAC tissue [3] |
| Corynebacterium spp. | 8 | EAC/ME tissue [3], PNS tissue [3], EAC swab [1], CSF [1] |
| Proteus spp. | 6 | EAC swab [4], EAC tissue [2] |
| E coli. | 3 | EAC swab [2], EAC tissue [1] |
| Klebsiella spp. | 3 | EAC swab [1], ME fluid [1], PNS tissue [1] |
| Enterococcus faecalis | 3 | EAC swab [1], EAC swab [1], tissue-masseter [1] |
| Strep. agalactiae | 2 | EAC swab [1], PNS tissue [1] |
| Propionibacterium acnes | 2 | Mastoid bone [1], mastoid tissue [1] |
| Morganella spp. | 2 | EAC swab [1], EAC tissue [1] |
| Citrobacter spp. | 1 | EAC swab [1] |
| Staphylococcus haemolyticus | 1 | Tissue-masseter [1] |
| Lactobacillus spp. | 1 | Tissue-masseter [1] |
| Prevotella spp. | 1 | EAC tissue [1] |
| NF gram negative | 1 | EAC swab [1] |
| No growth | 37 | Mastoid tissue [14], mastoid bone [7], PNS tissue [4], EAC/ME tissue [4], EAC swab [3], TMJ aspirate [2], mastoid swab [1], PNS swab [1], PNS aspirate [1] |
| Total | 183 | – |
EAC, external ear canal; ME, middle ear; TMJ, temporomandibular joint; PNS, postnasal space; CSF, cerebrospinal fluid.
Table 3
| Site of sample | Number of samples | Number positive | % positive |
|---|---|---|---|
| EAC | 32 | 30 | 94 |
| Postnasal space | 15 | 11 | 73 |
| Temporomandibular joint | 4 | 2 | 50 |
| Mastoid (bone, mucosa) | 19 | 5 | 26 |
SBO, skull base osteomyelitis; EAC, external ear canal.
Pseudomonas aeruginosa was the most frequently isolated microbe, present in 63/183 (34%) samples, collected from 39/63 (62%) patients. Staphylococcus aureus was the next most commonly isolated microbe, and a wide range of other bacterial species were isolated. Fungi were isolated from 38 samples from 23 patients. Seventy-one percent (27/38) of these isolates were cultured from swabs collected from the EAC. The remaining 11/38 (29%) isolates were cultured from EAC tissue, the postnasal space or the mastoid, in a total of 10 patients. These patients with positive fungal culture on tissue samples were considered to have fungal SBO.
Antimicrobial therapy was delivered principally by the IV route in 59 patients, and by the oral route in four patients, three who started oral treatment before returning to their country of origin for definitive IV treatment, and one who was a local patient who did not attend follow up. The most commonly administered IV therapies were ceftazidime (27 patients), and piperacillin/tazobactam (24 patients). Oral ciprofloxacin was administered in 30 patients, usually after IV antibiotic therapy had completed. The median duration of intravenous treatment was 42 days (range, 4–122 days), and the median duration of intravenous plus oral treatment was 75 days (range, 15–309 days). Mean IV treatment duration between those that had clinical resolution and those that had clinical relapse or failure did not differ significantly (40.93 and 40.14 days respectively) (P=0.16).
Three patients suffered drug reactions. Two were from Tazobactam/Piperacillin (cutaneous eruptions), one of which made an early transition to oral ciprofloxacin and the other who developed a rash in the last few days of treatment. One patient had a reaction from ceftazidime (pancytopaenia) whose treatment was terminated early, and who later relapsed.
Long-term outcome data was not available for 7 patients, 5 who were lost to follow up and 2 who died of unrelated illnesses. Follow up data was available for 56 patients who had a median of 145 days (range, 15–769 days) follow-up after the completion of treatment.
Clinical outcomes
Clinical outcomes are presented in Figure 2. Fifty percent (28/56) patients were cured a mean of 94 days (range, 24–257 days) after presentation, and 16/56 (28%) patients suffered a relapse of disease at a median of 51 days (range, 14–292 days) after the completion of IV treatment. All 16 patients who were followed up and observed to relapse were cured by a second course of treatment.
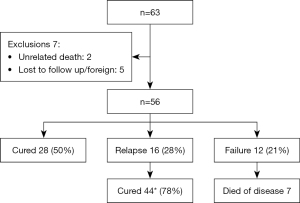
Disease specific mortality occurred in 7 (12.5%) of patients at a median of 49 days (range, 15–462 days) from presentation. Previously described determinants of severe disease (2) are listed with their association with clinical outcome in Table 4. Age over 70, CT scan erosion, and diabetes significantly increased the odds of clinical failure over clinical resolution. Facial palsy did not have a significant association with adverse outcome in our cohort. Lower cranial nerve palsy was seen in 5 patients at presentation; 2 who had clinical failure, 2 whose disease relapsed, and 1 who was clinically cured.
Table 4
| Predictor of severe disease | Clinical outcome | |
|---|---|---|
| Relapse | Failure | |
| Age >70 | 0.10 (0.01–1.05); P=0.55 | 3.49 (0.38–7.32); P=0.007 |
| CT scan erosion | 0.08 (0.01–1.06); P=0.55 | 4.13 (0.46–5.87); P=0.02 |
| Diabetes | 0.03 (0.001–0.90); P=0.04 | 0.03 (0.001–0.975); P=0.05 |
| Facial palsy | 0.30 (0.06–1.56); P=0.15 | 0.38 (0.06–2.52); P=0.32 |
CI, confidence interval; CT, computed tomography.
Radiologic outcomes
A gallium scan was appropriately performed at the end of the treatment course in 27 patients at a median of 17 days (range, 0–169 days) after the last treatment dose. Twelve patients who were clinically cured also had concordant radiographic resolution (true negative). Two patients had clinical cure but had radiographic evidence of ongoing SBO at the conclusion of treatment (false positive). Three patients suffered relapse of disease after post treatment scanning showed resolution of disease in the skull base (false negative). No patients that had clinical failure had radiographic resolution at the conclusion of treatment. Ten patients had concordant residual or progressive disease on post treatment gallium scan, and an adverse clinical outcome (failure or relapse).
The sensitivity and specificity of an abnormal gallium scan after a course of treatment to predict an unfavourable outcome (relapse or failure) was 76.9% and 85.7% respectively.
Inflammatory outcomes
The CRP was measured in 55 patients within 7 days either side of finishing treatment. The CRP was <5 in 18/28 (64%) patients who were clinically cured, and in 10/16 (63%) patients who went on to suffer clinical relapse (P=0.49). The median CRP in the week before death in the seven patients who died of SBO was 51 (range, 21–148). At the conclusion of treatment, neither inflammatory resolution nor radiographic resolution were observed in those who had clinical failure (Table 5).
Table 5
| Variable | Cure (%) | Relapse (%) | Failure (%) | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| Radiographic resolution# | 64 | 31 | 0 | 0.17 | 0.003 | – |
| Inflammatory resolution (CRP)# | 72 | 67 | 0 | 0.78 | 0.001 | – |
| Smoking^ | 18 | 12.5 | 17 | – | – | 0.33 |
^, recorded at first presentation; #, measured at the conclusion of treatment. P1, cure vs. relapse; P2, relapse vs. failure; P3, cure vs. relapse vs. failure. CRP, C-reactive protein.
Discussion
The incidence of SBO continues to increase in our region, and reliable control of this stubborn disease remains challenging. The median age, male predilection, and diabetes prevalence was similar to those seen in other literature (2,19) (Table 1). Our Pasifika population continues to be significantly overrepresented, which is concordant with prevalence of other chronic diseases amongst this group in New Zealand (20). The Pasifika population continues to grow in New Zealand, particularly in the Auckland region, and are approximately twice as likely to have diabetes mellitus than other ethnic groups in New Zealand (including Māori). These factors likely contribute in part to the higher rate of disease observed.
We discovered a relative abundance of positive cultures from the central skull base, and a relative paucity in the lateral skull base/mastoid despite the latter lighting up on nuclear medicine imaging. The prime locations for culture collection appear to be the EAC, and the central skull base. A cortical mastoidectomy alone for sampling should be carefully considered, given the paucity of meaningful culture yield from this site. The central skull base and postnasal space however, was likely to harbour useful infected tissue. Biopsy here was chosen when the EAC and/or mastoid biopsies were negative and the initial technetium scan was positive in this region. This was accessed using transnasal endoscopic techniques. Medial penetration of disease to the paranasopharyngeal soft tissue as previously described (21) was a more common route of spread than posterior spread to the mastoid. In one case, medial spread of infection was observed in serial imaging to ultimately reach the contralateral mastoid. The large variety of organisms cultured across the cohort supports culture driven treatment after appropriate sampling of infected tissue. Given the morbidity and mortality of the disease we believe it is appropriate to commence empiric anti-pseudomonal IV treatment while cultures are pending. Prolonged empiric antibiotic treatment must be reserved for the uncommon case of negative tissue cultures. In the case of culture negative SBO, if the patient improves on systemic antimicrobial treatment, no further biopsies are necessary and the treatment is completed as per protocol. If improvement (clinical and inflammatory marker reduction) is not seen, further biopsies for culture, or a change in antimicrobial is considered in consultation with an infectious disease physician.
A striking finding in this study was the high proportion who returned with relapse of disease after the initial treatment was completed (28%). Every case that was observed to relapse achieved cure after either a further prolonged treatment course, or alternative antibiotic agent. This observation suggests our current ability to confirm cure is suboptimal, and we believe supports a longer initial course of treatment. Such an extended course of treatment (beyond six weeks) in an attempt to obtain cure also makes pragmatic sense in the Australasian context, where less than half of patients with SBO attended for follow up scanning in a rural Australian setting (22). Seven patients died of disease after a median of 49 days of treatment. One of these patients had surgical debridement of necrotic tissue in the mastoid and 4 others underwent general anaesthesia for tissue culture, where no significant necrosis or abscess formation was evident. Forty-three percent (3/7) of those who died of disease had fungal SBO evidenced by fungal growth on tissue culture, and none were culture negative. SBO carries a risk of mortality despite appropriate antibiotic treatment, particularly those with significant comorbidity.
Reliable prediction of sustained clinical cure remains a challenge. Resolution of gallium scan uptake in the skull base, and normalisation of the CRP trended towards predicting clinical cure over relapse of disease; but neither reached statistical significance (Table 5).
We concur with recent literature that the utility of gallium scan alone to predict clinical outcome is limited. van Kroonenburgh (21) states no single imaging modality is suited to monitoring this condition, and advocates for both a functional and an anatomical radiographic assessment in the follow up of SBO. MRI can illustrate soft tissue improvement in infection/inflammation but bone marrow changes can linger for many months after disease thus limiting its ability to detect osteomyelitis resolution (23). FDG positron emission tomography (PET) may have superior specificity to Gallium 67 and other nuclear medicine studies for disease monitoring; while exposing the patient to approximately 1/3 the radiation exposure at a reduced cost (24,25). Unfortunately, large parts of New Zealand and Australia do not have easy access to such advanced imaging techniques so its inclusion into a treatment protocol may not be feasible.
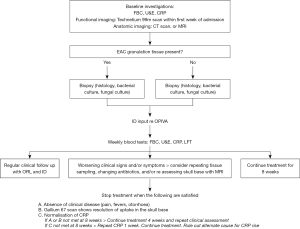
Predicting clinical failure is easier than predicting success or relapse. Because no patients that had clinical failure had radiographic resolution or inflammatory resolution, we advocate for ongoing IV antimicrobial treatment with infectious disease input in these cases, and repeat cultures as described in our updated protocol (Figure 3).
This review is limited by its retrospective nature, and a reliance on historical documentation to deduce clinical outcomes. A treatment protocol was introduced in 2011 by the department of otolaryngology, part way through the time of observation; perhaps contributing inconsistency in the treatment delivered across the cohort. Despite this, strict adherence to the protocol after its introduction was limited to only 23 patients (43%). This low adherence could be in part due to the decentralization of follow up of these patients across the wider Auckland region, and low adoption of the protocol by those not involved or aware of its genesis.
Conclusions
SBO is increasing in frequency the Auckland region, and there is a significant overrepresentation of Pasifika people amongst those affected. Obtaining representative infected tissue for diagnosis and culture is critical to facilitate culture directed treatment. The EAC and central skull base are favoured sites for sampling.
Some predictors of severe disease as previously described are useful to identify those that are more likely to fail treatment (age >70 years, diabetes, bony erosion on initial CT scan) but these predictors are not useful in identifying those that initially respond to treatment; but later relapse after treatment is stopped.
Confirming disease cure also continues to be problematic. Gallium scans and inflammatory markers are consistently abnormal in the patient that fails treatment but cannot be relied on alone to identify the patient that will relapse with disease. Hence a combination of radiologic, inflammatory, and clinical resolution is needed to best confirm cure.
The high relapse rate after treatment, and excellent response to re-treatment we believe supports a longer initial course of IV treatment; which has been adopted in our revised treatment protocol.
Acknowledgments
We wish to thank Dr. Monish Maharaj for support with SPSS statistical software use.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/10.21037/ajo-22-23/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-22-23/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-22-23/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-22-23/coif). AB reports a conflict of interest as Trainee Representative for ASID (Australasian Society of Infectious Diseases) from January 2021 to May 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Auckland Health Research Ethics Committee (AH25092). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chandler JR. Malignant external otitis. Laryngoscope 1968;78:1257-94. [Crossref] [PubMed]
- Stern Shavit S, Soudry E, Hamzany Y, et al. Malignant external otitis: Factors predicting patient outcomes. Am J Otolaryngol 2016;37:425-30. [Crossref] [PubMed]
- Mahdyoun P, Pulcini C, Gahide I, et al. Necrotizing otitis externa: a systematic review. Otol Neurotol 2013;34:620-9. [Crossref] [PubMed]
- Cunha CB, Opal SM. Antibiotic Stewardship: Strategies to Minimize Antibiotic Resistance While Maximizing Antibiotic Effectiveness. Med Clin North Am 2018;102:831-43. [Crossref] [PubMed]
- Singh A, Al Khabori M, Hyder MJ. Skull base osteomyelitis: diagnostic and therapeutic challenges in atypical presentation. Otolaryngol Head Neck Surg 2005;133:121-5. [Crossref] [PubMed]
- Carlton DA, Perez EE, Smouha EE. Malignant external otitis: The shifting treatment paradigm. Am J Otolaryngol 2018;39:41-5. [Crossref] [PubMed]
- Peled C, El-Seid S, Bahat-Dinur A, et al. Necrotizing Otitis Externa-Analysis of 83 Cases: Clinical Findings and Course of Disease. Otol Neurotol 2019;40:56-62. [Crossref] [PubMed]
- Rubin J, Yu VL. Malignant external otitis: insights into pathogenesis, clinical manifestations, diagnosis, and therapy. Am J Med 1988;85:391-8. [Crossref] [PubMed]
- Narozny W, Kuczkowski J, Mikaszewski B. Infectious skull base osteomyelitis--still a life-threatening disease. Otol Neurotol 2006;27:1047-8. [Crossref] [PubMed]
- Rubin Grandis J, Branstetter BF 4th, Yu VL. The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis 2004;4:34-9. [Crossref] [PubMed]
- Lazzarini L, Lipsky BA, Mader JT. Antibiotic treatment of osteomyelitis: what have we learned from 30 years of clinical trials? Int J Infect Dis 2005;9:127-38. [Crossref] [PubMed]
- Carfrae MJ, Kesser BW. Malignant otitis externa. Otolaryngol Clin North Am 2008;41:537-49. viii-ix. [Crossref] [PubMed]
- Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc 2011;86:156-67. [Crossref] [PubMed]
- Loh S, Loh WS. Malignant otitis externa: an Asian perspective on treatment outcomes and prognostic factors. Otolaryngol Head Neck Surg 2013;148:991-6. [Crossref] [PubMed]
- Honnurappa V, Ramdass S, Mahajan N, et al. Effective Inexpensive Management of Necrotizing Otitis Externa Is Possible in Resource-Poor Settings. Ann Otol Rhinol Laryngol 2019;128:848-54. [Crossref] [PubMed]
- Moss WJ, Finegersh A, Narayanan A, et al. Meta-analysis does not support routine traditional nuclear medicine studies for malignant otitis. Laryngoscope 2020;130:1812-6. [Crossref] [PubMed]
- Spielmann PM, Yu R, Neeff M. Skull base osteomyelitis: current microbiology and management. J Laryngol Otol 2013;127:S8-12. [Crossref] [PubMed]
- Statistics New Zealand: New Zealand Government. Available online: www.stats.govt.nz
- Stevens SM, Lambert PR, Baker AB, et al. Malignant Otitis Externa: A Novel Stratification Protocol for Predicting Treatment Outcomes. Otol Neurotol 2015;36:1492-8. [Crossref] [PubMed]
- Affairs. SNZaMoPI. Health and Pacific peoples in New Zealand. Statistics New Zealand and Ministry of Pacific Island Affairs 2011.
- van Kroonenburgh AMJL, van der Meer WL, Bothof RJP, et al. Advanced Imaging Techniques in Skull Base Osteomyelitis Due to Malignant Otitis Externa. Curr Radiol Rep 2018;6:3. [Crossref] [PubMed]
- Loh TL, Renger L, Latis S, et al. Malignant otitis externa in Australian Aboriginal patients: A 9-year retrospective analysis from the Northern Territory. Aust J Rural Health 2019;27:78-82. [Crossref] [PubMed]
- Singhal A, Sotoudeh H, Chapman PR. Skull base osteomyelitis imaging. Curr Opin Otolaryngol Head Neck Surg 2021;29:333-41. [Crossref] [PubMed]
- Sturm JJ, Stern Shavit S, Lalwani AK. What is the Best Test for Diagnosis and Monitoring Treatment Response in Malignant Otitis Externa? Laryngoscope 2020;130:2516-7. [Crossref] [PubMed]
- Stern Shavit S, Bernstine H, Sopov V, et al. FDG-PET/CT for diagnosis and follow-up of necrotizing (malignant) external otitis. Laryngoscope 2019;129:961-6. [Crossref] [PubMed]
Cite this article as: Emanuel H, Neeff M, Thomas M, Bryce A. Skull base osteomyelitis in the Auckland region. Aust J Otolaryngol 2023;6:2.


