
Delayed post-contrast MRI in the assessment of Meniere’s disease—initial South Australian experience
Introduction
Meniere’s disease (MD) is a chronic disease affecting the inner ear characterised clinically by intermittent episodic vertigo, fluctuating sensorineural hearing loss, tinnitus and aural fullness (1,2).
Establishing the diagnosis of MD is challenging in patients when their symptoms fall outside of definable criteria for diagnosis. There is also significant overlap in clinical findings of MD, vestibular migraine and benign paroxysmal positional vertigo which further confounds straightforward diagnosis. In 2015, the AAO-HNS criteria for stratification of patients into definite or probable MD was proposed, revised from the previous diagnostic criteria in 1995 (3-5).
The underlying pathology of MD has long been attributed to endolymphatic hydrops (EH), a post-mortem finding of enlargement of the endolymphatic structures due to increased volume of endolymph at the expense of perilymphatic spaces (1). EH may however represent an epiphenomenon, a final common endpoint of various pathophysiologic processes rather than the consequence of an isolated abnormality. However, the presence of EH does not always correlate clinically, with EH being also demonstrated in asymptomatic patients (6,7).
Previously the role of magnetic resonance imaging (MRI) in investigating patients with MD has been that of excluding other pathologies such as vestibular schwannoma or labyrinthitis. Recently, there has been a considerable interest in utilising MRI for visualisation of radiologic endolymphatic hydrops (rEH) in cases of suspected MD, allowing for radio-pathologic correlation.
We describe our early experiences with MRI imaging of suspected cases of MD and correlate the radiologic findings with the patient’s clinical presentation.
Methods
Ethics
This retrospective cross-sectional study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the local institution (Central Adelaide Local Health Network, No. EC00192) approved the study with waiver of informed consent.
Patients
A retrospective study was conducted of all consecutive adult patients presenting with audio-vestibular symptoms to a single neuro-otologist (SC) in an outpatient clinic in South Australia, who subsequently underwent MRI for assessment of rEH over an 18-month period between August 2018 and March 2020. All patients who underwent MRI during the study time frame were included in the study.
Imaging technique
All studies were performed on a 3T MRI (Siemens, Magnetom Skyra, Erlangen, Germany) using a 32 channel head coil at a private radiology clinic. Images were acquired 4 hours post intravenous administration of 15 mL Gadovist gadobutrol 9.071 g/15 mL. Imaging protocol consisted of heavily T2 weighted SPACE and 3D FLAIR sequences, with post-processed subtraction images using the technique described by Naganawa et al. (2,8) (Table 1, Figure 1).
Table 1
| Sequence | Parameters |
|---|---|
| T2 SPACE | • FOV 196 mm |
| • Slice thickness 1 mm | |
| • TR 4,490 ms | |
| • TE 540 ms | |
| • Flip angle 120 degrees | |
| • Scan time: 3:24 | |
| Post-contrast heavily T2-weighted 3D FLAIR | • FOV 196 mm |
| • Slice thickness 1 mm | |
| • TR 9,000 ms | |
| • TE 540 ms | |
| • TI 225 ms | |
| • Flip angle 120 degrees | |
| • Scan time: 15:11 | |
| Subtraction images | • The T2 SPACE sequence is subtracted from the heavily T2 weighted FLAIR image |
SPACE, Sampling Perfection with Application optimized Contrast using different flip angle Evolution; FOV, field of view; TR, repetition time; TE, echo time; FLAIR, fluid attenuated inversion recovery; TI, inversion time.
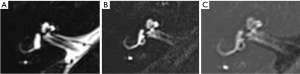
Image interpretation
De-identified images were independently reviewed by two radiologists (GL, TN) in a single reading session on the InteleViewer (InteleRad, Montreal QC, Canada) PACS system. The readers were blinded to clinical information.
Peri-lymphatic hyperenhancement was assessed as present or absent with use of a region of interest (ROI) tool.
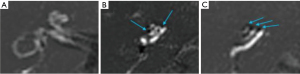
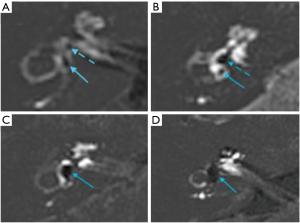
The assessment of rEH was based upon the classification system described by Bernaerts et al. (9) using a 3 point scoring system for cochlear hydrops (normal/0, 1, 2) (Figure 2), and a 4 point system for vestibular hydrops (normal/0, 1, 2, 3) (Figure 3). Any inter-reader discrepancies were resolved by consensus for comparison with clinical data.
Clinical assessment
Patient demographics, laterality, nature and duration of symptoms were recorded. Patients were stratified into “probable” or “definite” diagnoses of MD following clinical assessment by a single otolaryngologist (SC). A further category of “unlikely MD” was included for patients with non-specific symptoms not meeting the diagnostic criteria of MD, but when it was still considered as a differential diagnosis.
Statistical analysis
Statistical analysis for inter-observer variability in detecting rEH on MRI was performed with Cohen’s Kappa test (Stata Statistical Software: Release 15.1 College Station, TX: StataCorp LP). The Fisher’s exact test was utilised to assess the correlation between the clinical diagnosis of MD and MRI detection of hydrops (SAS 9.4 statistical software package SAS Institute Inc., Cary, NC, USA).
Results
Over a period from August 2018 to March 2020, 37 patients with mean age 50.7 years (range, 20–74 years), M:F 16:21, were clinically evaluated and underwent 3T MRI of the inner ear. In total, 74 ears were evaluated. Eighteen ears were clinically diagnosed with definite MD, 12 with probable MD and 44 with unlikely MD. Fourteen percent (5/37) of patients were diagnosed with bilateral MD (definite or probable). rEH was detected on MRI in a total of 23 ears (Table 2). Bilateral rEH was detected in 6/37 (16%) of patients. Sixty percent (15/25) of patients with a diagnosis of definite or probable MD demonstrated asymmetric perilymphatic enhancement on MRI.
Table 2
| Clinical diagnosis | Hydrops present | Hydrops absent | Total (n=74 ears) |
|---|---|---|---|
| Definite MD | 15 (83%) | 3 (17%) | 18 |
| Probable MD | 3 (25%) | 9 (75%) | 12 |
| Unlikely MD | 5 (11%) | 39 (89%) | 44 |
MRI, magnetic resonance imaging; rEH, radiologic endolymphatic hydrops; MD, Meniere’s disease.
Inter-observer agreement calculated using Cohen’s Kappa scores was 0.89 for the presence of rEH, 0.81 for the grading of vestibular hydrops and 0.76 for the grading of cochlear hydrops.
Correlation between imaging and clinical diagnosis
Fifteen out of 18 ears with clinically definite MD had rEH (83%). Three out of 12 ears with probable MD had rEH (25%). Five out of 44 ears with clinically unlikely MD had rEH (11%) (Table 2). There was a statistically significant correlation between the diagnosis of MD and the presence of rEH on MRI (Fisher’s Exact test P value <0.0001). Patients with a clinical diagnosis of definite MD were 39 times more likely to have rEH compared to a those with a diagnosis of unlikely MD (P<0.0001).
The average MRI grading score for cochlear hydrops was 0.14 for ears with unlikely MD, 0.33 for ears with probable MD, and 1.00 for ears with definite MD. The average MRI grading score for vestibular hydrops was 0.11 for ears with unlikely MD, 0.63 for ears with probable MD and 1.44 for ears with definite MD.
Discussion
MD often presents as a triad of symptoms including fluctuating sensorineural hearing loss, tinnitus and episodic vertigo. Patients may complain of aural fullness as well. Arriving at the diagnosis within the framework of AAO-HNS criteria is quite straightforward when individuals present with this well-recognised array of symptoms. While a host of audio-vestibular tests, including electrocochleography, have been used as an aid to the clinical assessment these tests are limited by their modest sensitivity and poor clinical correlation (10). In spite of the lack of availability of unequivocal diagnostic tests, the ability of the physician to diagnose MD with confidence is relatively high owing to the characteristic combination of symptoms, allowing the physician to focus on management strategies to improve the patient’s quality of life.
Nonetheless, a number of patients present with symptoms that are variable in both the frequency and the duration, as well as lack concordance between the vestibular and auditory symptoms (11). This has resulted in the use of “atypical MD” (12) as a diagnostic entity reflecting the clinical presentations which fall outside the AAO-HNS diagnostic criteria and are difficult to assign an alternative diagnosis but demonstrate some of the features of MD.
The need to arrive at a working diagnosis in individuals presenting with episodic imbalance assumes significance when we consider vestibular migraine as a relatively common differential diagnosis of MD. Like MD, the diagnosis of vestibular migraine also relies on a set of clinical criteria in the absence of unequivocal laboratory or radiologic abnormalities. Moreover, the management strategies for MD and vestibular migraine are divergent. Invasive (ablative) treatment can be considered in recalcitrant MD but not in vestibular migraine, making diagnostic certainty important.
EH is the pathologic correlate of MD. However, it is considered an epiphenomenon rather than a hallmark of this clinical syndrome since EH has been demonstrated in individuals without symptoms of MD (13). A plethora of cellular and local homeostatic aberrations may result in the development of EH (6). This is reflected in the diverse range of mechanisms proposed by various researchers to explain the aetiopathogenesis of MD (6). Nevertheless, in individuals presenting with the clinical symptoms consistent with MD, one may expect to find pathologic changes of EH (1).
Until recently, it was only possible to demonstrate the presence of EH by histopathologic examination of the otic capsule at autopsy. Current protocols for contrast enhanced MRI have been utilised for over a decade and reliably demonstrate the presence of EH owing to the differential distribution of the contrast medium in the perilymphatic and endolymphatic spaces of the inner ear, which is accentuated in patients with MD attributable to increased permeability of the blood labyrinthine barrier (14). This modality has afforded clinicians the opportunity to demonstrate the presence of rEH noninvasively, allowing correlation of changes in the membranous labyrinth with their clinical symptoms. MRI imaging techniques for evaluation of rEH have evolved, with a shift from intra-tympanic gadolinium (IT-gad) to intra-venous gadolinium (IV-gad) administration (2). The intra-venous route has the advantages of being less invasive and the ability to image both ears simultaneously for comparison (2), regardless of status of middle ear health and the variable anatomy of the round window niche. In addition, the interval between contrast injection and image acquisition is reduced to 4 hours for IV-gad, compared to 24 hours for IT-gad administration (2).
The authors’ imaging protocol includes a T2 SPACE sequence to assess for the presence of any non-MD pathologies which may contribute to the patient’s symptoms (such as vestibular schwannoma) and to allow for anatomic correlation on subsequent sequences.
Heavily T2-weighted 3D FLAIR sequences are superior to conventional post-contrast T1 sequences and provide excellent contrast discrimination between the hypointense endolymph and hyperintense perilymphatic structures (2,15) (Figure 1). The addition of post processed subtraction images enhances discrimination between the endolymph (black signal) and the adjacent otic capsule bone (grey signal), further improving specificity. A limitation of the 3D FLAIR sequence is the long scan duration predisposing to motion artefact and misregistration on the subtraction sequences, which may hinder interpretation.
A number of methods have been proposed for grading rEH. We utilised the grading system proposed by Bernaerts et al.; a 3-point system for cochlear hydrops and a 4-point system for vestibular hydrops (9) (Figures 2,3) and demonstrated excellent interobserver variability, similar to previously published results (9,16,17). This incorporates an additional vestibular grade to the system proposed by Baráth et al. (16), characterised by saccular enlargement without utricular conglomeration (saccule to utricle ratio inversion—SURI), initially described by Attyé et al. (17) (Figure 3B). Typically the saccule is the first vestibular endolymphatic structure to become hydropic, and represents the earliest imaging manifestation of vestibular hydrops (grade 1). Addition of the fourth vestibular grade results in improved sensitivity for detection of rEH compared to the three grade method, without compromising specificity (9). In our cohort, the presence of SURI was also useful for detection of MD; 3 ears with SURI as the sole radiologic finding had clinically definite MD. Asymmetric perilymphatic hyperenhancement, even in isolation without hydrops is reported as an ancillary feature strongly supportive of MD (9). This is reflected in our findings—12/15 (80%) of patients with perilymphatic hyperenhancement had definite MD.
In our study, 15/18 (83%) of clinically diseased ears were diagnosed with rEH on MRI, comparable to the results of Baráth et al., who detected rEH in 55/61 (90%) of diseased ears. Of the false negatives, one included a patient in whom MD was suspected bilaterally, but rEH was only detected in one ear. In 4 out 5 cases where rEH was demonstrated in clinically normal ears, the patient had rEH and clinical MD in the contralateral ear. This is not unexpected and is consistent with other studies demonstrating rEH on MRI in 22% of clinically normal ears, likewise, EH has been demonstrated histopathologically in 5–26% of asymptomatic patients (7,18). Whether rEH is a precursor to clinical MD and will eventually lead to symptoms requires further evaluation with longitudinal studies.
A proportion of patients with peripheral vestibulopathy may not present with symptoms that are typical of MD. Patients may retain some characteristics of MD, however a clinical diagnosis based on the AAO-HNS diagnostic criteria is not possible. Atypical MD is a well-recognised clinical entity (19) and can be associated with delay in diagnosis and treatment. In our cohort, there were 12 patients who presented with atypical symptoms and were stratified into the “probable MD” group. This group included patients with a short duration of symptoms, vestibular-only or auditory-only symptoms, and symptoms overlapping with vestibular migraine. Of these, 3 patients (25%) had rEH on MRI. It is our opinion that presence of rEH can aid the clinician in arriving at a provisional diagnosis for management of patients presenting with atypical audio-vestibular symptoms.
Delayed contrast enhanced MRI of the inner ear for hydrops is a relatively inexpensive test. In Australia, it is a Medicare rebatable examination similar to the commonly performed contrast enhanced examinations of the internal acoustic meati assessing for vestibular schwannomas, at a cost of approximately $350 to the Medicare system. Gadolinium is a safe contrast agent with limited risks in the healthy population when utilised judiciously with appropriate clinical indications. The risk of anaphylaxis and serious drug reactions is extremely low, reported in <0.1% of cases (20). Nephrogenic systemic fibrosis is a rare progressive multi-organ fibrosing disorder occurring in patients with severe renal failure (estimated glomerular filtration rate of 30 mL/min/1.73 m2) following IV gadolinium administration, with an estimated incidence of less than 0.07% (21). At our institution, all patients who may receive IV gadolinium are screened for renal impairment, and gadolinium administration is contraindicated in those found to have severe renal dysfunction. More recently, there has been concern regarding the deposition of gadolinium within the intracranial neuronal tissues, which has been confirmed histopathologically (22). However, this is thought to be a dose dependent phenomenon related to repeated gadolinium exposure rather a single dose, and as yet there have been no known short- or long-term adverse effects related to this deposition (23,24).
Limitations of the study include the small sample size and the retrospective nature of the study. Potential areas for future analysis include evaluating the relationship between MR imaging findings with duration/severity of symptoms, and with clinical response to treatment.
Conclusions
Delayed post-contrast MRI with heavily T2 weighted 3D FLAIR sequences is a useful tool for detection of rEH, with particular utility in confirming the diagnosis in patients with atypical symptoms.
Acknowledgments
The authors would like to thank James Hancock and Dallas Quigley (Benson Radiology) for implementing the MRI protocols and Suzanne Edwards (University of Adelaide) for statistical support.
Footnote
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-21-27/dss
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-21-27/coif). The authors have no conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the local institution (Central Adelaide Local Health Network, No. EC00192) approved the study with waiver of informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gürkov R, Pyykö I, Zou J, Kentala E. What is Menière’s disease? A contemporary re-evaluation of endolymphatic hydrops. J Neurol 2016;263:S71-S81. [Crossref] [PubMed]
- Naganawa S, Nakashima T. Visualization of endolymphatic hydrops with MR imaging in patients with Ménière’s disease and related pathologies: current status of its methods and clinical significance. Jpn J Radiol 2014;32:191-204. [Crossref] [PubMed]
- Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Menière’s disease. J Vestib Res 2015;25:1-7. [Crossref] [PubMed]
- Goebel JA. 2015 Equilibrium Committee Amendment to the 1995 AAO-HNS Guidelines for the Definition of Ménière’s Disease. Otolaryngol Head Neck Surg 2016;154:403-4. [Crossref] [PubMed]
- Basura GJ, Adams ME, Monfared A, et al. Clinical Practice Guideline: Ménière’s Disease. Otolaryngol Head Neck Surg 2020;162:S1-S55. [Crossref] [PubMed]
- Salt AN, Plontke SK. Endolymphatic hydrops: pathophysiology and experimental models. Otolaryngol Clin North Am 2010;43:971-83. [Crossref] [PubMed]
- Rauch SD, Merchant SN, Thedinger BA. Meniere’s syndrome and endolymphatic hydrops. Double-blind temporal bone study. Ann Otol Rhinol Laryngol 1989;98:873-83. [Crossref] [PubMed]
- Naganawa S, Kawai H, Taoka T, et al. Improved HYDROPS: Imaging of Endolymphatic Hydrops after Intravenous Administration of Gadolinium. Magn Reson Med Sci 2017;16:357-61. [Crossref] [PubMed]
- Bernaerts A, Vanspauwen R, Blaivie C, et al. The value of four stage vestibular hydrops grading and asymmetric perilymphatic enhancement in the diagnosis of Menière’s disease on MRI. Neuroradiology 2019;61:421-9. [Crossref] [PubMed]
- Güneri EA, Çakır A, Mutlu B. Validity and Reliability of the Diagnostic Tests for Ménière’s Disease. Turk Arch Otorhinolaryngol 2016;54:124-30. [Crossref] [PubMed]
- Wladislavosky-Waserman P, Facer GW, Mokri B, et al. Meniere’s disease: a 30-year epidemiologic and clinical study in Rochester, Mn, 1951-1980. Laryngoscope 1984;94:1098-102. [Crossref] [PubMed]
- Kato M, Sugiura M, Shimono M, et al. Endolymphatic hydrops revealed by magnetic resonance imaging in patients with atypical Meniere’s disease. Acta Otolaryngol 2013;133:123-9. [Crossref] [PubMed]
- Gluth MB. On the Relationship Between Menière’s Disease and Endolymphatic Hydrops. Otol Neurotol 2020;41:242-9. [Crossref] [PubMed]
- Pakdaman MN, Ishiyama G, Ishiyama A, et al. Blood-Labyrinth Barrier Permeability in Menière Disease and Idiopathic Sudden Sensorineural Hearing Loss: Findings on Delayed Postcontrast 3D-FLAIR MRI. AJNR Am J Neuroradiol 2016;37:1903-8. [Crossref] [PubMed]
- Carfrae MJ, Holtzman A, Eames F, et al. 3 Tesla delayed contrast magnetic resonance imaging evaluation of Ménière’s disease. Laryngoscope 2008;118:501-5. [Crossref] [PubMed]
- Baráth K, Schuknecht B, Naldi AM, et al. Detection and grading of endolymphatic hydrops in Menière disease using MR imaging. AJNR Am J Neuroradiol 2014;35:1387-92. [Crossref] [PubMed]
- Attyé A, Eliezer M, Boudiaf N, et al. MRI of endolymphatic hydrops in patients with Meniere’s disease: a case-controlled study with a simplified classification based on saccular morphology. Eur Radiol 2017;27:3138-46. [Crossref] [PubMed]
- Merchant SN, Adams JC, Nadol JB Jr. Pathophysiology of Meniere’s syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol 2005;26:74-81. [Crossref] [PubMed]
- Hannigan IP, Rosengren SM, Young AS, et al. A Portrait of Menière’s Disease Using Contemporary Hearing and Balance Tests. Otol Neurotol 2022;43:e489-e496. [Crossref] [PubMed]
- Franckenberg S, Berger F, Schaerli S, et al. Fatal anaphylactic reaction to intravenous gadobutrol, a gadolinium-based MRI contrast agent. Radiol Case Rep 2018;13:299-301. [Crossref] [PubMed]
- Woolen SA, Shankar PR, Gagnier JJ, et al. Risk of Nephrogenic Systemic Fibrosis in Patients With Stage 4 or 5 Chronic Kidney Disease Receiving a Group II Gadolinium-Based Contrast Agent: A Systematic Review and Meta-analysis. JAMA Intern Med 2020;180:223-30. [Crossref] [PubMed]
- Ayers-Ringler J, McDonald JS, Connors MA, et al. Neurologic Effects of Gadolinium Retention in the Brain after Gadolinium-based Contrast Agent Administration. Radiology 2022;302:676-83. [Crossref] [PubMed]
- Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834-41. [Crossref] [PubMed]
- Gulani V, Calamante F, Shellock FG, et al. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 2017;16:564-70. [Crossref] [PubMed]
Cite this article as: Nguyen TT, Lim GS, Chawla S. Delayed post-contrast MRI in the assessment of Meniere’s disease—initial South Australian experience. Aust J Otolaryngol 2023;6:7.


